Chapter Outline
Congenital Anomalies of the Pancreas
To recognize the anatomic variants of the pancreas and to understand how various pancreatic anomalies develop, it is important to be familiar with pancreatic embryology and development. Errors or variations at several critical periods in the development of the pancreas are responsible for the majority of anomalies. These anomalies can vary in their presentation from being totally asymptomatic to being inconsistent with life. In this chapter, a brief discussion of the important events of pancreatic embryology is followed by presentation of normal variants and various types of pancreatic anomalies.
Embryology
The pancreatic duct develops from two buds originating from the endodermal lining of the duodenum. One is the dorsal pancreatic bud, which is in the dorsal mesentery and is seen as a diverticulum of the foregut before 28 days. It grows into the dorsal mesentery ( Fig. 96-1A ). The other is the ventral pancreatic bud located close to the bile duct and appears as an invagination at the biliary-duodenal angle between 30 and 35 days.

The dorsal and ventral pancreatic buds soon grow into a pair of branching, arborized ductal systems, each with its own central duct ( Fig. 96-1B ). At day 37, the ventral pancreas rotates posterior to the duodenum and comes into contact with the dorsal pancreas ( Fig. 96-1C ). These two anlagen fuse and together with the duodenum fuse with the abdominal wall. In the mature organ, the ventral primordium becomes the inferior portion of the head and the uncinate process, and the dorsal pancreas becomes the body and tail. After the fusion, a new duct connects the distal portion of the dorsal pancreatic duct with the shorter duct of the ventral pancreas to form the main duct or the duct of Wirsung ( Fig. 96-2 ). This main pancreatic duct, which is present in approximately 91% of adults, enters the duodenum together with the bile duct at the major papilla. The proximal portion of the dorsal pancreatic duct is pinched off during fusion and usually atrophies and disappears but may persist as the small accessory duct of Santorini, which has a variety of appearances ( Fig. 96-3 ). This accessory duct empties into the duodenum at the minor papilla 2 to 3 cm proximal to the ampulla of Vater. In about 10% of all cases, the duct system fails to fuse and the original double system persists.


As the duodenum grows and differentiates, the duodenal wall resorbs the distal bile duct up to its junction with the pancreatic duct. Different degrees of resorption account for variations in the appearance and relationships of the common bile duct and pancreatic duct. If ductal resorption is minimal, a long intramural ampulla is created, and the junction is extramural. The junction becomes intramural with increasing degrees of resorption, which produces a shortened ampulla. Maximal resorption produces separate orifices for the pancreatic duct and common bile duct (see Fig. 96-2 ), which no longer share a common ampulla.
Beginning in the third month of fetal life, the islets of Langerhans develop as clusters of cells from the terminal ductules. They become intimately associated with the capillary plexus and finally separate from the ductules to become the endocrine portion of the pancreas. Insulin secretion begins at approximately the fifth month. The acini develop from terminal ductal cells. The ductal system and the acini collectively become the exocrine portion of the pancreas. Secretory activity probably becomes established in the pancreas during the second trimester, although this has been disputed. The weight of the pancreas, which is 5 to 5.5 g at birth, will increase to 15 g at the end of the first year.
The process of pancreatic fusion is complicated, and a wide spectrum of anomalies or anatomic variants related to this process may appear—for example, agenesis, aplasia of the pancreatic anlage, hypoplasia, annular pancreas, and pancreas divisum.
Variants of Normal Anatomy
The lateral aspect of the head and neck of the pancreas can have varieties of shapes and occasionally look prominent ( Fig. 96-4 ). However, many of the contour variations may represent a spectrum of fusion patterns that cannot be attributed to pancreas divisum alone. A deep cleft separating two distinct pancreatic moieties may be identified on computed tomography (CT) scans in the head and neck of the pancreas in patients with pancreas divisum.

The pancreas is surrounded by fat that clearly defines its margin. However, because of the lack of any pancreatic capsule, the pancreatic lobules can be outlined by fat. Such fatty infiltration can be diffuse or focal ( Fig. 96-5 ). Focal fatty infiltration can mimic a mass on CT, particularly if there is associated lobulation (see Fig. 96-4 ). Therefore, magnetic resonance imaging (MRI) may become necessary to differentiate this benign process from a neoplasm ( Fig. 96-6 ). Fatty infiltration may be associated with focal sparing of pancreatic parenchyma, and that should not be mistaken for tumor.


The position and configuration of the pancreas are variable, and these variations may simulate pathologic conditions. For example, the pancreatic head is not fixed in position, although it almost invariably maintains a fixed relationship medial to the second portion of the duodenum and lateral to the root of the superior mesenteric vessels, even if these structures are shifted to the left of the midline. Although the splenic vein usually marks the dorsal margin of the body and tail of the pancreas, the tip of the gland may rarely curve dorsal to the splenic vein to simulate adrenal abnormalities ( Fig. 96-7 ). On occasion, even in normal persons, the pancreatic tail may be anterior-lateral to the left kidney, where it may appear as a pseudomass on excretory urography. In patients with prior left nephrectomy or in those with congenital absence of the left kidney, the pancreatic tail is often displaced into the renal fossa, which may simulate recurrent tumor or a primary retroperitoneal lesion.

Kreel and colleagues published a set of in vivo and in vitro measurements of pancreatic dimensions and concluded that the normal diameter of the head is up to 3 cm, that of the neck and body up to 2.5 cm, and that of the tail up to 2 cm. However, the size, shape, and position of the normal pancreas are highly variable ( Fig. 96-8 ), and there is usually a gradual tapering from the head to the tail without abrupt alterations in size or contour. There is gradual decrease in the size of the pancreas with advancing age, sometimes becoming very small beyond the seventh decade. Fatty lobulations are more commonly observed in obese and elderly individuals. Rarely, an accessory spleen can be embedded in the tail of the pancreas, and MRI or a nuclear medicine study may be needed to differentiate this variant from a mass ( Fig. 96-9 ).


Bifid pancreatic duct ( Fig. 96-10 ) is a rare anatomic anomaly in which the main pancreatic duct is bifurcated along its length. It is associated with a high incidence of pancreatitis.

Congenital Anomalies of the Pancreas
Pancreas Divisum
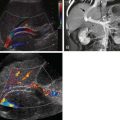
In this anomaly, the pancreas is divided in two separate parts as a result of an absent or incomplete fusion of the ventral and dorsal anlagen. As a consequence, the pancreatic head and uncinate process are drained by the duct of Wirsung through the major papilla; the body and tail are drained by the duct of Santorini through the minor papilla ( Fig. 96-11 ). This anomaly is seen in 4% to 11% of autopsy series and 3% to 4% of endoscopic retrograde cholangiopancreatography (ERCP) series. In an analysis of 650 ERCP studies done by Uomo and coworkers, 485 patients had satisfactory imaging of the pancreatic ducts, whereas in 48 cases (9.9%), anatomic variants of the duct were found. These included fusion variants in 26 cases (22 pancreas divisum and 4 functional divisum) and duplication variants in 22 patients (1 bifurcation of the main pancreatic duct, 4 loop, 2 N shaped, and 3 ring).

Clinical Findings
Although the anatomic variant of pancreas divisum has been well known for some time, its clinical significance has become evident with the advent of ERCP. Most cases of pancreas divisum are asymptomatic. This anomaly may contribute to recurrent episodes of idiopathic pancreatitis in younger patients with no risk factors. Between 12% and 26% of patients with idiopathic recurrent pancreatitis have this anomaly, as opposed to 3% to 6% in the general population. The age at presentation varies widely but is most commonly between 30 and 50 years. Several reports of pancreatitis associated with pancreas divisum in children have appeared, and there is a report of this anomaly occurring in multiple family members.
It is postulated that in pancreas divisum, the duct of Santorini and its accessory ampulla are too small to adequately drain the volume of secretions produced by the pancreatic body and tail. In a manometer study, patients with pancreas divisum had significantly higher pressure readings of the dorsal duct cannulated through Santorini than of the ventral duct cannulated through Wirsung. The study concluded that in pancreas divisum, there is chronic stasis of pancreatic fluid that, compounded by additional factors such as alcoholism, causes greater viscosity, which increases the risk of pancreatitis. Surgical sphincteroplasty of the accessory sphincter has been advocated as an important means of preventing recurrent bouts of pancreatitis in these patients.
Association of pancreatic divisum with pancreatic tumors has been reported. In a limited study, pancreatic tumors were detected in 12.5% of patients with divisum. The authors believed that relative stenosis of the minor pancreatic duct and long-standing pancreatic obstruction might be risk factors for development of pancreatic cancer.
There are some other problems associated with divisum, like santorinicele or cystic dilation of the dorsal pancreas at the minor papillae, that may indicate obstruction at the minor papilla. Also, cases of multiple neuroendocrine tumors of the pancreas and intestinal malrotation have been reported in association with pancreas divisum.
Radiologic Findings
Endoscopic Retrograde Cholangiopancreatography.
ERCP is often considered the effective modality for confirming a diagnosis of pancreas divisum. Often, injection of contrast material into the duct of Wirsung is met with resistance and even pain. However, after opacification, the duct is short and tapered, and acinarization commonly occurs as the endoscopist attempts to fill the “remainder” of the duct. This duct tapers gradually from the orifice, sending branches in the pancreatic head. It must be differentiated from a duct that appears foreshortened secondary to previous trauma, partial pancreatectomy, pseudocyst, or stricture caused by carcinoma or pancreatitis. In these cases, the pancreatic duct typically has an abrupt or irregular terminus. The duct of Santorini is often not visualized by injection of the major papilla, and if cannulation is successful, the full-length duct of Santorini is seen without communication to the smaller duct. Otherwise, the diagnosis can be made by secretin stimulation and identification of secretions emanating from the minor papilla only. See Chapter 74 for more details.
Linear Array Endoscopic Ultrasonography.
Endoscopic ultrasonography (EUS) frequently allows detailed imaging of the pancreatic parenchyma and ductal system. It has been reported that with the use of radial instruments, if the portal vein, common bile duct, and main pancreatic duct could be demonstrated in one image, the diagnosis of pancreatic divisum would be excluded. This is considered an indirect assessment for the evaluation of pancreatic divisum. Therefore, with a linear array instrument, if the main pancreatic duct passes from the major papilla to the pancreatic body and tail, or if the duct crosses the border separating the ventral and dorsal anlagen, the diagnosis of pancreatic divisum is excluded. Lai and associates reported the sensitivity, specificity, and accuracy of this method for diagnosis of pancreas divisum to be 95%, 97%, and 97%, respectively. There are some merits for EUS over magnetic resonance cholangiopancreatography (MRCP), and perhaps ERCP, as it allows direct visualization and fine-needle aspiration biopsy of pancreatic masses that otherwise may lead to the ductographic phenomenon of pseudodivisum. This could conceivably result from the presence of apparently separate ventral and dorsal ductal systems due to mechanical obstruction near the expected point of connection between these two systems. Also, EUS seems to be less invasive than ERCP because EUS does not involve pancreatic duct cannulation or injection of contrast material, which is a risk factor for development of iatrogenic pancreatitis. Therefore, linear array EUS appears to be a promising, minimally invasive diagnostic imaging modality for the detection of pancreas divisum.
Sonographic Secretin Test.
In an attempt to identify patients who will benefit from surgical sphincterotomy, several tests have been proposed to assess the adequacy of the accessory papilla in transmitting pancreatic secretions. In the sonographic secretin test, the main pancreatic duct is sonographically monitored before and after the intravenous administration of secretin (1 unit/kg body weight). Secretin-induced dilation occurs in 72% of symptomatic patients found to have a stenotic accessory papilla associated with pancreas divisum. The sonographic secretin test is also highly controversial; several authors have demonstrated that dilation of the pancreatic duct can be a normal finding. In addition to surgery, pancreas divisum can be treated by percutaneous dilation and stent placement.
Computed Tomography.
Contour abnormalities of the pancreatic head and neck have been identified on CT in some patients with pancreatic divisum. CT can occasionally suggest this diagnosis when two distinct pancreatic moieties or an unfused ductal system is identified on thin-collimation scans (see Fig. 96-11 ). The two moieties may cause pancreatic head enlargement or may be separated by a fat cleft. Sometimes, fatty replacement of the dorsal pancreas may delineate it from the ventral moiety. Also, the pancreatic head may be spared from atrophy and pancreatitis affecting the body and tail, which produces a pseudotumor on CT and ultrasound studies. In alcoholics, isolated ventral pancreatitis may occasionally be observed, which suggests a synergism between the effects of alcohol and bile reflux into the ventral pancreas. The dorsal pancreas is spared this reflux because of the pancreas divisum. Other ductal anomalies may also produce masses on cross-sectional imaging.
Multidetector CT with high-resolution oblique coronal image reconstruction assists in the depiction of the continuity of pancreatic ducts. The sensitivity and specificity of this method for diagnosis of pancreatic divisum are reported to be 100% and 89%, respectively.
Magnetic Resonance Cholangiopancreatography.
Heavily T2-weighted, two-dimensional, fast spin-echo sequences using a body coil can often accurately depict pancreatic ductal anomaly and establish the diagnosis of pancreas divisum. Gradient sequences using breath hold have been found useful for evaluation of the pancreatic and biliary system. MRCP is superior to ERCP for visualizing the pancreatic ducts, especially with the use of half-Fourier acquisition single-shot turbo spin-echo (HASTE) sequence. However, MRCP with single-shot rapid acquisition with relaxation enhancement may be superior to HASTE for increasing pancreatic duct conspicuity.
When T1-weighted sequences with fat suppression are performed, MRCP allows visualization of not only the pancreatic ducts but also the pancreatic parenchyma around the duodenum. Indeed, MRCP may be able to replace ERCP in the diagnosis of pancreas divisum.
Secretin-stimulated MRCP has been used in the diagnosis of santorinicele or cystic dilation of dorsal pancreas at the minor papillae and also for pancreas divisum (see Fig. 96-11 ). See Chapter 75 for more detail concerning MRCP.
Annular and Semiannular Pancreas
Annular pancreas is a rare congenital anomaly occurring in 1 of every 12,000 to 15,000 live births. In this anomaly, the annulus is often a flat band of pancreatic tissue completely encircling the second portion of the duodenum ( Figs. 96-12 and 96-13 ). Unusual locations of annular pancreas have also been reported to be around the third portion of the duodenum.