Endovascular aneurysm repair (EVAR) was initially introduced with the aim of achieving lower mortality and morbidity rates in elderly and unfit patients deemed to be at greater risk for complications from conventional surgery. Since its introduction by Parodi almost 2 decades ago, EVAR has become widely used worldwide and is now the main method used to treat many patients with AAAs, not only the elderly and unfit.1 Indications for treatment of abdominal aortic aneurysms are: • Aneurysm diameter. Treatment of AAAs is based on the aneurysm reaching a size threshold above which leaving it untreated is more hazardous than treatment. The treatment threshold is based on the perceived annual rupture rate of aneurysms of different diameters. Although rupture rates of small aneurysms are very low, the rate increases to 10% per year once aneurysms reach 6 cm in diameter. The U.K. Small Aneurysm Trial (SAT) and the U.S. Aneurysm Detection and Management (ADAM) study were conducted to discern optimal management of AAAs between 4.0 and 5.5 cm. Both studies concluded that surgery should be performed at a threshold diameter of 5.5 cm, and that surgery for smaller aneurysms provided no survival advantage. It remains controversial whether the 5.5-cm threshold should apply to women, and many interventionalists treat AAAs in women when they reach 5 cm, even less in some centers.2–4 • Symptomatic aneurysms. Painful or tender AAAs should be treated. • Rapidly growing aneurysms. A diameter increase of 5 mm or greater in 6 months is recognized as an indication for intervention. The most commonly used devices at this time are the Zenith endograft (Cook Medical, Bloomington, Ind.) (Fig. 47-1, A), Endurant endograft (Medtronic), AneuRx Endograft (Medtronic) (Fig. 47-1, B), Excluder endograft (W.L. Gore & Associates, Flagstaff, Ariz.) (Fig. 47-1, C). Other devices include the Aorfix endograft (Lombard Medical, Didcot, UK), Endologix Powerlink endograft (Edwards Life Sciences, Irvine, Calif.), and Anaconda endograft (Terumo Medical Corp., Somerset, N.J.). The following items are routinely used for implantation of these devices: • Single-piece arterial puncture needles • Vascular sheaths—6F to 18F, depending on the endograft selected • Guidewires—standard, hydrophilic, and ultrastiff guidewires in 180 cm and 260 cm lengths • Catheters—aortic flush and selective catheters • Balloons—large-caliber balloon catheters to dilate or mold the devices to the aortic neck or iliac arteries (e.g., Coda balloon [Cook Medical]) All patients require comprehensive imaging of the aorta and iliac arteries to assess their suitability for EVAR. The mainstay of imaging is computed tomography (CT), which provides optimal information on the lumen and wall of the aortoiliac segment. Magnetic resonance imaging (MRI) is used preferentially by some centers and is useful in patients who are allergic to iodinated contrast material. Images should be assessed in the axial plane and also via multiplanar reconstruction using maximum intensity projection images.5 • The procedure may be performed in the angiography suite or operating room. The most important aspect is the quality of the imaging equipment, which outweighs potential benefits in terms of sterility provided by operating rooms. • The best results of EVAR are achieved by using a team approach consisting of interventional radiologists and vascular surgeons. Each team member contributes different complementary skills to the procedure, enabling even the most difficult problems to be overcome. • Most procedures are performed under general anesthesia, although regional and local anesthesia are options. • Access to the arterial system is usually achieved by bilateral femoral arteriotomies. However, percutaneous closure devices are now available for closure of large-caliber access site punctures, and many interventionalists now use these in preference to conventional femoral arteriotomies in suitable patients. • In most cases, the main body is inserted via the right femoral artery, and the contralateral limb is passed up the left femoral artery. Operators may decide to pass the main body up the left side if the right side is severely diseased and unsuitable for passage of the large-caliber main body delivery system. • The left femoral artery is punctured, and a 6F sheath is inserted. Via this access, a standard aortic flush catheter is advanced into the abdominal aorta and placed with the catheter tip at the level of L1. • The right femoral artery is catheterized, and a selective catheter (e.g., Cobra) and a standard guidewire are advanced into the upper thoracic aorta. The standard guidewire is exchanged for an exchange-length very stiff guidewire (e.g., Lunderquist [Cook Medical]), and the catheter is removed. • The main body is advanced over the Lunderquist guidewire to the level of L1 (to the approximate level of the renal arteries). A flush aortogram is performed with a small amount of contrast medium (e.g., 7-10 mL injected at 10 mL/s). The aim of aortography is to locate the origins of the renal arteries. The CT scan should be scrutinized to assess whether any angulation of the intensifier is required (in either the oblique or craniocaudal projection) to profile the origins of the renal arteries. In general, adequate opacification of the aorta and renal arteries can be achieved with these small amounts of contrast medium. Many patients have impaired renal function, so efforts should be made to limit the amount of contrast medium. Carbon dioxide is a very effective contrast medium for aortography and can be used in lieu of iodinated contrast for all or many parts of the procedure. • The top of the main body is deployed so that the graft material is positioned immediately below the renal arteries. After deployment of the main body, repeat aortography may be performed to confirm the correct location of the endograft and continued patency of the renal arteries. • The next step is catheterization of the contralateral limb. The pigtail catheter (which is outside the graft) is withdrawn over a guidewire into the lower aorta and exchanged for an angled-tip catheter such as a Cobra, Vanshee (Cook Medical), or Berenstein (Cordis Corp., Warren, N.J.) catheter. With the aid of an angled-tip hydrophilic guidewire and/or oblique angulation of the image intensifier, the operator cannulates the opening in the main body for the contralateral limb. This step may be straightforward or extremely difficult. Factors that cause difficulty in cannulation include a large aneurysm with little mural thrombus, tortuous iliac arteries, and angulation of the aneurysm in the anteroposterior plane. • In some cases, retrograde cannulation of the contralateral limb opening may not be possible. The contralateral limb opening can be cannulated antegradely by snaring a wire passed through the opening from the ipsilateral side. • The contralateral limb is inserted so that the top of the limb overlaps the markers in the contralateral limb opening on the main body. The aim is to deploy the limb so that it lands just proximal to the common iliac bifurcation. If the graft is a type that has limb sections bilaterally (e.g., Zenith), the ipsilateral limb is inserted after the contralateral limb. Limb extensions may be placed if there is a large distance between the lower end of a limb and the common iliac bifurcation. • If patients have aneurysmal common iliac arteries that cannot provide a sealing zone for the endograft limbs, it is necessary to extend the limbs into the external iliac arteries. Embolization of the internal iliac artery (IIA) is required before this maneuver to prevent retrograde leaks up the IIA after the procedure. Alternatively, branched iliac endografts are now available and may be used in preference to IIA embolization. • All attachment sites and connections are dilated with large-caliber compliant balloons. • Finally, completion angiography is performed to assess patency of the endograft, iliac arteries, and renal arteries and absence of a leak into the aneurysm sac. • It is important to perform completion angiography with all stiff guidewires removed because kinks or stenoses of the limbs may be concealed if the limbs are straightened out by the presence of stiff guidewires. The usual technique is to place a flush catheter up one iliac limb of the device, and leave a catheter or balloon up the other limb so that access to the endograft is maintained from each side of the groin to manage any problems revealed by angiography. • If type 1 or type 3 endoleaks are visible on completion angiography, they should be treated immediately (see later discussion). • If completion angiography suggests a kink in one of the endograft limbs as it passes around a curve in the iliac artery, or angulation of the tip of the limb with respect to its attachment to the native iliac artery, the kink or the angulated end of the graft should be supported by the insertion of a suitable uncovered self-expanding stent. • Most but not all AUI devices are two-piece devices with an upper body and a lower limb that are designed to be placed just above the ipsilateral common iliac bifurcation. An occluder or vascular plug is placed in the contralateral common iliac artery to prevent an endoleak from occurring as a result of retrograde flow up the common iliac artery. • The first part of the procedure is exactly the same as for bifurcated devices until the proximal component of the device has been deployed. • The distal component is inserted and deployed. The site of the ipsilateral common iliac artery bifurcation is defined by angiography via the pigtail catheter that is left in situ after deployment of the proximal endograft. • The pigtail catheter is withdrawn over a guidewire, and a Lunderquist guidewire is passed up the contralateral femoral access so that the tip is placed in the aneurysm sac. The sheath containing the occluder is advanced up the contralateral iliac artery, and the occluder is extruded so that it is deployed in the proximal common iliac artery. Two prospective randomized trials for the evaluation of EVAR for small AAAs have recently reported their findings. The PIVOTAL trial (Positive Impact of Endovascular Options for Treating Aneurysms Early) is a U.S. multicenter trial in which 728 patients with 4.0- to 5.4-cm AAAs were assigned to either ultrasound (US) surveillance or early EVAR with the AneuRx endograft.6
Aortic Endografting
Clinical Relevance
Indications
Equipment
Endografts
Equipment Required for Implantation
Technique
Anatomy and Approach
Imaging
Technical Aspects
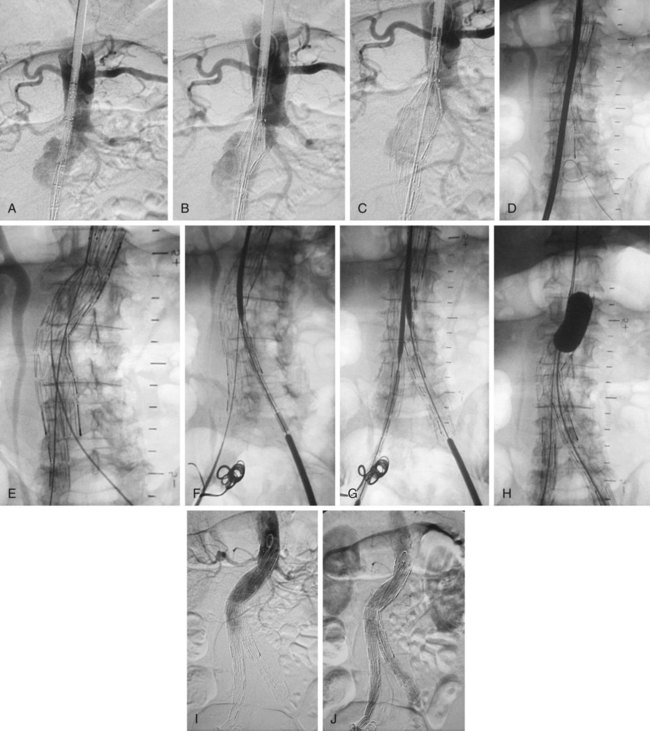
Procedure for Device Insertion (Bifurcated Devices) (Fig. 47-2)
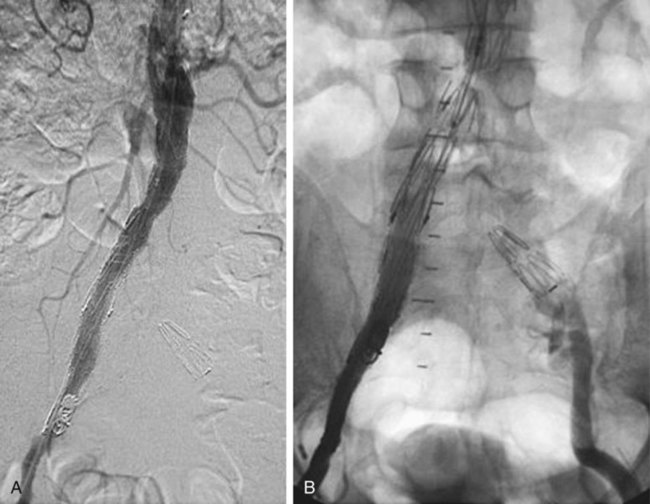
Insertion of Aortouniiliac Devices (Fig. 47-3)
Controversies
Should Patients with Aneurysms Smaller Than 5.5 cm Be Treated?
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Aortic Endografting