Over the past decade, stent-graft use for the treatment of aortic pathologies has steadily increased. Over 64% of infrarenal aortic aneurysms were treated with endovascular aneurysm repair (EVAR) in 2007.1 Expanded indications have occurred as additional experience has been obtained using this technology and includes use of stent-grafts to treat aneurysms in the pararenal and visceral aorta, as well as emergent and nonaneurysmal pathologies.2,3 This, however, has been associated with a rise in complication rates as devices are being implanted outside their intended “instructions for use” (IFU) guidelines.4–6 To meet this demand for greater application of endovascular techniques, medical device manufacturers have improved stent-graft design (Fig. 11-1) with new design modifications that are currently being evaluated in clinical trials. Hopefully, such trials will provide data to enable the clinician to apply newer devices to increasingly complex aortic pathologies that have traditionally been treated by open procedures.7 Current indications for stent-graft implantation in the infrarenal aorta involve the treatment of abdominal aortic aneurysms (AAAs). Strict indications include the presence of an AAA with a greater than 5 cm diameter, rapidly expanding aneurysms (>0.5 cm in 6 months), or aneurysms greater than two times the normal aortic diameter. The patient should also be a suitable candidate for endovascular exclusion based on anatomic criteria. Although these specifications differ slightly based upon which device is being implanted, they generally include the following characteristics. For an infrarenal aortic stent-graft, the aortic neck must be of suitable diameter and quality to provide an adequate seal. This is considered to be at least 10 mm in length, relatively free of significant calcification and thrombus, a nonaneurysmal neck (18-32 mm in diameter) with parallel walls, and angled less than 60 degrees. The anatomic limitations for the access vessels are less strict. Iliac stenosis, calcification, and tortuosity should allow for passage of the devices and have adequate length for sealing regions. Preservation of one hypogastric artery for pelvic blood flow is suggested, but isolated cases of bilateral internal iliac artery exclusion have been reported.8,9 New iliac-branched devices are in clinical trials in the United States and may help preserve hypogastric flow in patients who do not have an adequate sealing zone in the common iliac arteries.10 Aortic aneurysms in the region of the renal arteries are grouped collectively as pararenal aneurysms and further classified into juxtarenal and suprarenal subtypes. There is no universally agreed-upon definition of the term juxtarenal aneurysm, but it is commonly used to describe a complex AAA with either a short infrarenal neck or one that encroaches upon the renal segment of the aorta. Suprarenal aneurysms involve renal arteries and extend up to the splanchnic arteries. Type IV thoracoabdominal aortic aneurysms extend to a variable abdominal length but always involve the visceral aortic segment. Classification systems have been proposed, but none have gained wide acceptance or clinical use.11 This lends some ambiguity to the terms and makes comparison of clinical studies difficult. Until recently in the United States, the only way to treat pararenal aneurysms using endovascular techniques was to modify grafts to accommodate the anatomy of the individual patient or to use “off-label” current devices and technology. These techniques include handmade placements of fenestrations or scallops, openings in the graft material of the stent-graft to accommodate the visceral vessels, or use of a “snorkel” or “chimney” technique where stents are placed by upper extremity access through visceral vessels and extended superior to the graft material of an EVAR device to allow blood to flow to the visceral vessels. As of the writing of this chapter, there are two U.S. Food and Drug Administration (FDA)-approved clinical trials currently underway for fenestrated devices for treatment of juxtarenal aneurysms. The first trial, which is nearing completion (phase III), is the Cook Zenith fenestrated device (Cook Medical, Bloomington, Ind.). This device is indicated for use in aneurysms with a 4-12 mm neck and less than 45 degree of angulation at the neck.7 The second trial, beginning enrollment shortly, is from Endologix (Irvine, Calif.) for an “off-the-shelf” fenestrated device. Other aortic pathologies that have been treated with endovascular devices include ruptured AAA, penetrating atherosclerotic ulcers, mycotic aneurysms, aortoenteric fistulas, emboligenic lesions, and traumatic aortic injuries.2,12,13 Although not designed for these entities, many times the devices function extremely well, since there is an increased surface area contact with the device, thereby limiting device migration issues. However, limitations do exist based upon appropriate sizing and small aortic bifurcations. Long-term data regarding the treatment of infected pathologies is limited. Use of endovascular devices as a temporizing method in these emergent high-risk operations is appealing. Successful EVAR is directly related to appropriate patient selection. Preprocedural computed tomographic angiography (CTA) with axial and multiplanar reformatted imaging can adequately identify and delineate the patient’s anatomic characteristics. Axial imaging alone has some shortcomings with respect to accurately sizing tortuous anatomy.14 The equipment needed to perform EVAR involves typical interventional sheaths, wires, and catheters, with the addition of larger sheaths and longer and stiffer wires. Table e11-1 details appropriate equipment, but individual preferences can be substituted in many instances. Initial access wires and sheaths should include starter wires and 5F to 8F sheaths. Three grades of wire stiffness can be used, and in order of increasing stiffness include the Amplatz Superstiff, Meier, and Lunderquist wires. Each of these wires has its own inherent characteristics and can be applicable in different situations, depending on the interventionalist’s preferences and patient’s anatomy. Although some devices can be inserted over 180-cm-long wires, 260-cm or longer lengths are typically needed. Larger sheaths are sometimes necessary to help accommodate iliac tortuosity and should include sizes from 16F through 24F. Now that most devices, except the Gore Excluder and TAG (W.L. Gore & Associates, Flagstaff, Ariz.), are designed for sheathless insertion, sheaths are becoming less necessary. The presence of significant iliac tortuosity may require utilization of rigid sheaths to allow completion of the procedure. Occasionally, sequential dilators are necessary to determine whether iliac access is possible prior to opening a device. TABLE e11-1 Suggested Ancillary Equipment for EVAR and TEVAR EVAR, Endovascular aneurysm repair; TEVAR, thoracic endovascular aneurysm repair. When dealing with large sheaths in the iliac arteries, one important consideration is prolonged limb ischemia. These sheaths are commonly occlusive in not only the external iliacs but also the hypogastric arteries. In complex fenestrated or branched endovascular cases, it is important to minimize ischemia by careful planning and proceeding in an efficient manner. If a case is known to be lengthy, it may be worthwhile to place 5F sheaths in the common or superficial femoral arteries in an antegrade fashion. These sheaths can then be connected to the larger sheaths to maintain continuous blood flow and minimize ischemic time to the legs. Temporary axillobifemoral bypass has also been reported as an adjunct measure in these procedures to help minimize leg ischemia.15 When diseased or tortuous iliac arteries are present, additional maneuvers may be necessary to allow insertion of the delivery catheter. This can involve one of several techniques that includes stiffer or secondary “buddy” wire, dilatation or angioplasty of isolated stenosis, retrograde iliac endarterectomy, or placement of an iliac conduit. An endoconduit may also be performed, where a covered stent is placed inside the stenotic artery and ballooned to the appropriate size.16 Recanalization of occluded iliac arteries using subintimal angioplasty has also been reported. Each of these techniques contains individual risks and benefits that must be weighed in each patient.
Aortic Stent-Grafts
Clinical Relevance
Indications
Infrarenal Aneurysms
Pararenal Aneurysms
Contraindications
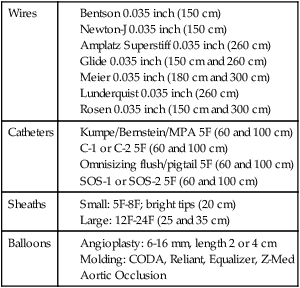
Equipment
Wires
Catheters
Sheaths
Balloons
Technique
Technical Aspects
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Aortic Stent-Grafts