- •
Connection between the pulmonary artery and the aorta above the level of the two semilunar valves.
- •
The window is typically large.
- •
Commonly seen in association with other anomalies such as interruption of the aortic arch, coarctation of the aorta, and tetralogy of Fallot.
Anatomy and Anatomical Associations
An aortopulmonary window (APW) is a communication, or natural connection, between the ascending aorta and the main pulmonary artery. The presence of two semilunar valves distinguishes this anomaly from a truncus arteriosus. Both semilunar valves are normal in position (except when APW is in the presence of other malformations such as tetralogy of Fallot). The pulmonary artery and the aorta are typically contiguous at the defect ( Figure 18-1 ). Generally, the connection occurs between the left margin of the ascending aorta and the right margin of the pulmonary artery. The defect is typically quite large and may be located a variable distance from the semilunar valves. A simple APW is defined as one without any significant associated anomalies, defects not requiring repair (such as a right aortic arch), or defects requiring a minor or simple repair (patent ductus arteriosus, atrial septal defect). A complex APW is one that occurs with another complex anomaly such as interruption of the aortic arch, tetralogy of Fallot, or anomalous origin of the coronary arteries. A variety of classification systems have been developed based on location and anatomical variations.

Coexisting cardiac anomalies have been reported in 47% to 77% of patients with an APW. Kutsche and Van Mierop reviewed 249 patients with an APW and found 52% of the patients had an associated congenital cardiovascular anomaly. The most common anomalies were type A interruption of the aortic arch or severe coarctation of the aorta (13%). Other reported lesions included a right aortic arch (9%), bicuspid aortic valve, ventricular septal defect (VSD), tetralogy of Fallot (6%), anomalous origin of the coronary arteries from the pulmonary trunk (5%), atrial septal defect, pulmonary valve stenosis, tricuspid valve atresia, and aortic valve atresia or stenosis. After birth a persistent patent ductus arteriosus has been reported in approximately 12%. Complete transposition of the great arteries and APW has also been reported. Berry’s syndrome consists of a distal APW, interrupted aortic arch, and aortic origin of the right pulmonary artery. Other extracardiac malformations have also been reported including anomalies of the musculoskeletal, central nervous, renal, pulmonary, and gastrointestinal systems. VATER (vertebral defects, imperforate anus, tracheoesophageal fistula, and radial and renal dysplasia) and VACTERL (vertebral abnormalities, anal atresia, cardiac abnormalities, tracheoesophageal fistula and/or esophageal atresia, renal agenesis and dysplasia, and limb defects) syndrome associations have also been reported in patients with APW.
Frequency, Genetics, and Development
APW is a rare congenital heart defect accounting for 0.2% to 0.6% of all congenital heart disease. The female-to-male gender ratio for this defect is 1 : 3.
APW is caused by defective development of conotruncal ridges that normally grow to partition the aorta from the pulmonary artery. The separation of the truncus arteriosus is incomplete or totally absent, leaving a persistent window between the aorta and the pulmonary artery. The aortopulmonary septum is formed by the two opposing truncal cushions that rapidly enlarge and fuse, dividing the truncus arteriosus into separate aortic and pulmonary channels. This division is influenced by cells that migrate from the neural crest. Removal of neural crest tissue results in other conotruncal abnormalities such as truncus arteriosus and transposition, but not APW, suggesting perhaps a different embryological origin.
APW is not typically a hallmark for chromosomal abnormalities. Despite the fact that the anomalies are in the same region within the heart, an APW appears to be pathogenetically unrelated to other conotruncal malformations such as truncus arteriosus. There is no known causative genetic abnormality associated with APW.
Prenatal Physiology
APW has very little impact on the fetus owing to the high pulmonary vascular resistance associated with fetal circulation. The defect can be identified in utero by visualization of the large communication between the aorta and the pulmonary artery. Flow patterns in the aorta, however, may be slightly altered. An APW can play the same role as a ductus arteriosus in the fetus, allowing for delivery of blood from the pulmonary artery to the aorta. Because the APW connects to the aorta more proximally than does the ductus arteriosus, the transverse aorta and isthmus may appear larger than normal because it receives antegrade flow from the left ventricle/ascending aorta as well as from the pulmonary artery via the APW. Because a greater degree of blood from the right ventricle is shunted to the aorta through the APW, the ductus arteriosus may be smaller than normal.
Prenatal Management
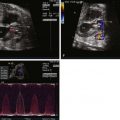
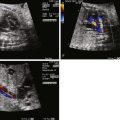
Prenatal diagnosis of APW is possible and has been described, although reports are limited. Because the pulmonary artery and aorta are contiguous, adjacent structures at the site of the APW connection, it can be difficult to make the diagnosis because the tissue planes are quite thin and the region in question can have false drop-out.
The APW connection can be seen wherever bifurcation of the pulmonary arteries are best seen and wherever there is contiguity between the aorta and the pulmonary artery. The short-axis view in particular can be helpful because it best aligns the planes between the aorta and the pulmonary artery. By angling from a short-axis view cephalad into the mediastinum, the aortopulmonary septum can be visualized between the aortic root and the bifurcation of the pulmonary arteries.
Postnatal Physiology
Infants with a simple APW are hemodynamically stable and frequently asymptomatic in the early postnatal period and, therefore, do not require an urgent neonatal intervention. Following the typical hemodynamic alterations of early infancy, the heart often becomes enlarged owing to increased shunting across the defect from aorta to pulmonary artery and volume load. The pulmonary arteries may also become enlarged as a result of increased flow.
The timing to identification of APW is often based on the presence and type of associated lesions. Those with ductal-dependent lesions such as interruption of the aortic arch will present very early in infancy. Those patients with a moderate-sized isolated APW will commonly present with symptoms within the first few weeks of life. These include signs of heart failure such as tachypnea, diaphoresis, and failure to thrive. Cyanosis is rare but can occur with bidirectional shunting through a large defect. On physical examination, there may be tachypnea, retractions, prominent right ventricular impulse, and bounding pulses (due to diastolic runoff). By auscultation, the second heart sound is accentuated and narrowly split. A prominent ejection click may also be heard in the pulmonic area. A loud systolic ejection murmur at the left upper sternal border or a machinery-type murmur is present. A thrill may be palpated. A middiastolic rumble may also be heard at the apex due to increased flow across the mitral valve. The anomaly may be confused with a patent ductus arteriosus. A large VSD and truncus arteriosus should also be considered in the differential diagnosis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree