Diffusion MR imaging exploits the diffusion properties of water to generate contrast between normal tissue and pathology. Diffusion is an essential component of nearly all brain tumor MR imaging examinations. This review covers the important clinical applications of diffusion weighted imaging in the pretreatment diagnosis and grading of brain tumors and assessment of treatment response. Diffusion imaging improves the accuracy of identifying treatment-related effects that may mimic tumor improvement or worsening. Fiber tractography models of eloquent white matter pathways are generated using diffusion tensor imaging. A practical and concise tractography guide is provided for anyone new to preoperative surgical mapping.
Key points
- •
Clinical diffusion weighted imaging exploits the random motion of water molecules to generate contrast between normal tissue and disease.
- •
Diffusion is a valuable sequence in the initial evaluation of intracranial tumors, which can narrow the differential diagnosis and increase diagnostic confidence.
- •
Noninvasive presurgical mapping of eloquent white matter by diffusion tractography can reduce operative complication rates.
- •
Interpreting radiologists should understand the common causes of nonvisualized fibers by clinical tractography to better guide surgery.
Introduction
Diffusion MR imaging is a powerful technique that exploits the diffusion properties of water to generate contrast between normal tissue and pathology. The relative restriction of random water movement (diffusion) in vivo is measured using paired diffusion sensitizing gradients, which causes greater signal loss in diffusing relative to stationary spins. Since its introduction 30 years ago, diffusion MR imaging has transformed the practice of radiology by demonstrating high sensitivity and specificity for ischemic, infectious, toxic-metabolic, and neoplastic conditions. In brain tumor imaging, diffusion MR imaging has been used to assess tumor type and grade, treatment response, and guide surgical intervention.
Diffusion weighted imaging (DWI) is the most basic implementation of diffusion MR imaging and assumes a simple but clinically useful model of isotropic gaussian diffusion. DWI is “diffusion weighted” because it is not a true map of diffusion but also contains T2 and other contrasts; therefore, at least one additional set of images with no or little diffusion weighting is also required to calculate the apparent diffusion coefficient (ADC), a semiquantitative measure that relates to the actual diffusion coefficient and removes the contribution of “T2 shine-through.”
Diffusion tensor imaging (DTI) expands on DWI by fitting a tensor model of diffusion, taking into account anisotropic diffusion in different directions and enabling calculation of metrics, such as fractional anisotropy (FA) ( Table 1 ). Furthermore, DTI has been used to produce models of white matter bundles in the brain, or fiber tractography (FT), which is useful for neurosurgical planning and intraoperative navigation. Although there is much exciting research in this area, a detailed physics review of advanced diffusion methods is beyond the scope of this article. The current clinical applications of diffusion imaging for evaluation of brain tumors is our focus.
| Metric | Definition | Notes |
|---|---|---|
| Axial diffusivity | Diffusion magnitude along the primary orientation of axonal fiber bundles | Largest or principal eigenvalue |
| Radial diffusivity | Diffusion magnitude perpendicular to the axonal fiber bundles | Average of the two minor eigenvalues |
| Mean diffusivity | Directionally averaged diffusivity of water in a voxel | Average of all 3 eigenvalues; equals ADC |
| Fractional anisotropy | Degree of coherent directionality of intravoxel diffusivity | Values from 0 to 1 0 = unrestricted 1 = linear |
Diffusion weighted imaging
Pretreatment
DWI is a useful sequence in the initial evaluation of intracranial tumors and tumor-like lesions. Restricted diffusion, as demonstrated by increased DWI signal and correspondingly reduced ADC, is caused by decreased free motion of water molecules, either because of high cellular density (eg, lymphoma and medulloblastoma) or high protein content (eg, epidermoid cyst). When used in conjunction with clinical history and other imaging features, DWI can help narrow the differential diagnosis and increase diagnostic confidence.
Extra-axial masses
Meningiomas are the most commonly encountered extra-axial lesion in clinical practice and can have a variable appearance on DWI. Qualitatively, some meningiomas can have slightly increased signal on DWI compared with the adjacent brain parenchymal. Lower ADC values have been reported to be associated with high-grade (atypical or malignant) meningiomas. Similarly, there are also preliminary reports of using ADC to differentiate between hemangiopericytomas from meningiomas (higher ADC in the former), but further study is needed to confirm these findings.
Dermoid and epidermoid cysts are along the histologic spectrum of ectodermal inclusion cysts and are classified depending on the presence of only epidermal component (epidermoid) or epidermal and dermal appendages (dermoid). Desquamated keratin in epidermoid cysts gives them the characteristic high DWI signal, enabling it to be readily differentiated from similar cystic lesions, such as arachnoid cyst. Dermoid cysts can have variable high DWI signal depending on amount of desquamated keratin, but they also have high T1 signal because of their lipid content. Practically, clear distinction between dermoid and epidermoid at imaging is not crucial, because it does not alter management of these related benign entities.
Intra-axial masses
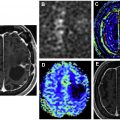
Diffusion imaging is helpful in the evaluation of pediatric and adult intra-axial tumors. In children, medulloblastomas and other embryonal tumors typically demonstrate high DWI signal and reduced ADC because of their high cellularity and nuclear-to-cytoplasmic ratio. This enables reliable distinction from other posterior fossa masses, such as ependymoma ( Fig. 1 ) and pilocytic astrocytoma, which tend to have intermediate or facilitated diffusion. Atypical teratoid/rhabdoid tumor is an aggressive embryonal tumor that also demonstrates restricted diffusion, but typically presents in a younger age group than medulloblastomas (<2 years vs mid to later childhood).

DWI plays a key role in the differentiation of gliomas and lymphomas, two of the most commonly encountered adult brain tumors. Central nervous system lymphoma are usually homogeneously hyperintense on DWI and has significantly lower ADC than glioblastoma, 0.630 ± 0.155 × 10 −3 mm 2 /s versus 0.963 ± 0.119 × 10 −3 mm 2 /s. Diffusion within glioma varies from intermediate to high and correlates with histologic grade, with higher-grade tumors, such as anaplastic astrocytomas and glioblastoma, demonstrating lower ADC than low-grade gliomas (World Health Organization I and II). Glioblastomas, in particular, are more heterogeneous than untreated lymphoma, with solid enhancing component demonstrating mild hyperintensity on DWI because of a combination of T2 shine-through and intermediate diffusion, and necrotic components demonstrating facilitated diffusion. Intratumoral hemorrhage is also more common in glioblastoma, and its associated susceptibility artifact can produce erroneous high DWI signal that must be recognized to avoid misinterpretation.
Other central nervous system tumors, especially those in the category of “small-blue-round-cell” tumors, such as primitive neuroectodermal tumor, germ cell tumor, and retinoblastoma, can demonstrate restricted diffusion. These are rarer and their characteristic locations can often provide helpful clues. Metastatic brain tumors can have variable appearance on DWI, depending on their primary source. Some have reported that the peritumoral edema of metastatic lesions have higher ADC than the nonenhancing component of gliomas, presumably because of higher cellularity of the latter.
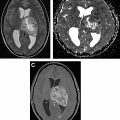
Nonneoplastic processes, such intracranial abscess and tumefactive demyelination, can often mimic tumor and demonstrate restricted diffusion. In contrast to lymphoma and other tumors, however, it is the central necrotic component of the abscess that demonstrates intense restricted diffusion rather than the solid cellular portion. Intracranial abscesses also tend to have relevant history and laboratory findings that aid in diagnosis. However, tumefactive demyelination can pose a diagnostic dilemma and appear similar to brain tumors, but its diffusion restriction is often along a “front” of active demyelination, occurring in a characteristic incomplete rim rather than a solid mass ( Fig. 2 ).

Post-treatment
A challenge in post-treatment imaging of brain tumors is the differentiation of treatment effects, from true tumor progression. Diffusion imaging can play a helpful role in this task when interpreted with detailed knowledge of tumor type and treatment history. In the immediate postsurgical setting, devitalized tissue along the resection cavity is expected to demonstrate restricted diffusion. Sometimes, however, the extent of restricted diffusion can extend beyond the surgical cavity and may involve large vascular territories, raising concern for perioperative infarction. This is more common when the mass is close to or encases vital vascular structures. Susceptibility artifact associated with postsurgical blood products can produce apparent hyperintensity on DWI and must be differentiated from true restricted diffusion.
Radiation therapy induces complex changes to the tumor and surrounding brain, which is dependent on tumor genetics, radiation dose, concurrent chemotherapy, and time. Paradoxic worsening of conventional image findings (enhancement and FLAIR) can occur within the first 3 months of completion of chemoradiation of high-grade gliomas, especially among tumors with IDH-1 mutation and methylation of the MGMT promoter. This phenomenon is termed pseudoprogression and reflects an inflammatory response to tumor necrosis and connotes improved survival. A retrospective series evaluated the role of ADC ratio (minimal ADC in lesion/contralateral normal-appearing white matter) in differentiation between pseudoprogression and true progression, and found diagnostic accuracy of 86.7% when using ADC ratio alone, and 93.3% when used in a multiparametric model combined with dynamic susceptibility contrast perfusion and MR spectroscopy. Interval increase in ADC after stereotactic radiosurgery of brain metastases has also been shown to be predictive of pseudoprogression, with accuracy of 77% according to one report.
Radiation necrosis commonly occurs 3 months to a year following radiation and may be delayed for up to multiple years. Differentiation of radiation necrosis and tumor progression is difficult based on conventional imaging appearance, and addition of DWI has been shown to improve diagnostic accuracy. A recent study showed that addition of ADC cutoff of 1 × 10 −3 mm 2 /s to Brain Tumor Reporting and Data System improved its diagnostic performance either alone (area under the curve, 0.76–0.88), or in combination with dynamic susceptibility contrast (area under the curve, 0.92). Complex techniques that consider heterogeneity within a lesion, such as volume-weighted voxel-based multiparametric clustering of diffusion and perfusion features, improved diagnostic accuracy over single imaging parameters. With the growth of machine learning and radiomics, there will undoubtedly be newer algorithms that incorporate conventional and advanced imaging features to classify tumor outcome. However, heterogeneity within treated tumor itself, where radiation necrosis often coexists with viable tumor, poses a challenge to the development and implementation of automated techniques.
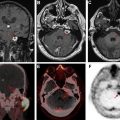
The vascular endothelial growth factor inhibitor bevacizumab is commonly used in recurrent glioblastoma. This medication has antiangiogenic effects and reduces blood-brain barrier permeability, thereby masking the enhancement of viable tumor (pseudoresponse). Restricted diffusion can occur in either the tumor bed or elsewhere in the brain, presumably caused by ischemic necrosis ( Fig. 3 ). Restricted diffusion in malignant gliomas treated with bevacizumab that is persistent over time is usually associated with good outcomes. However, pathologic studies have shown that progressive bevacizumab-induced restricted diffusion is associated with coagulative necrosis surrounded by viable tumor and associated with poor outcomes. Thus, it is important to evaluate serial changes in diffusion restriction on follow-up imaging and to incorporate all clinical and imaging data available to make the most accurate assessment.

Diffusion tensor imaging
Pretreatment
To preserve functional tissue, state-of-the-art MR imaging preoperative mapping of brain tumor patients has become the standard of care across the country. Neuroradiology-generated preoperative mapping of eloquent white matter tracts helps the neurosurgeon balance morbidity with resecting as much tumor as possible, which correlates with improved patient survival. The emergence of DTI with FT has allowed neurosurgeons to resect tumors with decreased complication rates , and increased median survival rates.
Knowledge of the technical basis of DTI combined with white matter anatomy is essential in the application of preoperative FT. DTI uses the anisotropic diffusion of water along coherently organized white matter tracts. FA value of 1 means that diffusion happens along one axis and is restricted in other directions (ie, axonal bundle). Most clinical DTI protocols use at least 30 diffusion-encoded image sets at b = 1000 s/mm 2 along noncollinear directions in addition to b = 0 s/mm 2 image set with 2- to 3-mm isotropic voxels to create the diffusion tensor. Deterministic FT generates streamlines, originating from user-defined seed points, along the dominant diffusion direction of the tract in three-dimension (3D) from voxel to voxel. Tracking parameters can then be manipulated to constrain the streamlines to anatomically represent eloquent white matter pathways. Various technical aspects of FT have been discussed previously and are an essential review for anyone new to brain mapping.
FT data are coregistered with volumetric structural sequences using various Food and Drug Administration–approved post-processing software for surgical navigation. The “buttonology” of these platforms varies, but the concepts of seed placement and tract titration are consistent. For nonenhancing tumors, it is paramount to fuse the tensor data with a 3D-FLAIR or T2 sequence. For enhancing lesions, postcontrast 3D-T1 images should be included. Seeds are placed in the plane perpendicular to the known trajectory of the desired tract to obtain as many streamlines as possible. A seed point can be drawn on several adjacent slices as another method to capture more streamlines. Our review focuses on two white matter tracts most frequently requested by our neurosurgical colleagues (corticospinal tract [CST] and arcuate fasciculus [AF]). Using real examples, we describe relevant anatomy, seed placement, and appropriate tract titration. A brief discussion on the limitations of clinical deterministic FT is included.
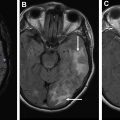
Motor: corticospinal tract
The CST or pyramidal tract is responsible for controlling movement in the contralateral torso, upper and lower extremities. It descends from the precentral gyrus and converges through the posterior limb internal capsule before traversing the brainstem. It decussates at the caudal medulla and connects with spinal motor neurons. Using the direction-encoded color FA maps as a guide, the first seed is drawn in the axial plane to cover the cerebral peduncle ( Fig. 4 A). Cover as much of the peduncle as possible to ensure obtaining the CST fibers. The purpose of the second seed point is to constrain the tract to match known anatomy. The second seed is placed on axial images to cover the precentral gyrus subcortical white matter just inferior to the level of the hand knob ( Fig. 4 B). In the case of the CST the second seed point excludes the frontal pontine and parietal pontine fibers ( Fig. 4 C).