Introduction
Patients presenting with undifferentiated shortness of breath can be challenging because their presentations can have many potential etiologies: pulmonary, cardiac, mixed cardiopulmonary, gastrointestinal, neurologic, or other noncardiopulmonary reasons. It is important to evaluate all organ systems potentially involved using a standardized, structured fashion to reach helpful conclusions rapidly at the bedside.
Preceding chapters have explored and discussed organ-based evaluations utilizing ultrasound. This chapter will focus on integrating those applications into combined algorithmic approaches to undifferentiated shortness of breath.
Ultrasound protocols have been described for evaluation of a variety of symptoms and diagnostic concerns. Perhaps the most well-known is the focused assessment with sonography for trauma (FAST) examination looking for free fluid in the setting of trauma, which was expanded to the eFAST examination to assess for traumatic pneumothorax. Other well-known protocols that include assessments for dyspnea include the focused assessment with sonography for HIV-associated tuberculosis (FASH) and the rapid ultrasound in shock (RUSH) examination for hypotension.
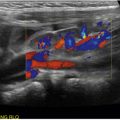
Examples of specific algorithms for the ultrasound evaluation of undifferentiated dyspnea include the bedside lung ultrasound in emergency (BLUE) protocol and the rapid assessment of dyspnea with ultrasound (RADiUS) protocol. The BLUE protocol evaluates the lungs only and is useful for the diagnosis of pneumothorax, pulmonary edema, pulmonary consolidation, and pleural effusions. The RADiUS protocol includes echocardiographic and inferior vena cava (IVC) evaluation, in addition to the pulmonary examination. A fluid administration limited by lung sonography (FALLS) protocol combines cardiac and lung views to sequentially rule out obstructive and cardiogenic shock while expediting diagnosis of distributive (usually septic) shock and monitoring for development of pulmonary edema as a result of fluid resuscitation.
Most algorithms combine the cardiac examination (including the IVC) with a thoracic examination, culminating in a “focused chest” assessment, as many life-threatening etiologies of dyspnea have cardiopulmonary origins. This approach is similar to the methods for evaluation of undifferentiated chest pain and hypotension described in other chapters.
Technique
The sequence for obtaining views varies, depending on the pretest probability of certain diagnoses obtained from the history and physical examination. Many sonologists choose to start with echocardiographic views, as the heart is essential.
Cardiac
The “5Es” approach is a problem-driven algorithm. The assessment for pathology is largely qualitative.
- •
Effusion—Is there an effusion? Is there pericardial tamponade?
- •
Parasternal long or short axis, apical four-chamber, or subxiphoid views
- •
- •
Ejection (fraction)
- 1.
Is the left ventricular function normal or depressed? Is congestive heart failure a reason for dyspnea?
- •
Parasternal long or short axis, apical four-chamber or subxiphoid views
- •
- 2.
Is there acute focal wall motion abnormality, indicating acute myocardial infarction with dyspnea as a symptom equivalent?
- •
Parasternal short axis, complemented by parasternal long axis or apical four-chamber view
- •
Equality—Is the right ventricle enlarged? Is there acute right heart strain from obstructive pathology such as a large pulmonary?
- •
- •
Parasternal long or short-axis, apical four-chamber views
- •
Exit—Is there thoracic aortic dilation? Is an aortic catastrophe causing the shortness of breath?
- •
- •
Parasternal long-axis view specifically measuring the aortic outflow tract (AOT)
- •
Entrance—What is the volume status (preload) entering the heart? Plethora and collapsibility of the IVC contribute to the interpretation of wall motion abnormalities and obstructive pathologies. Is hypovolemia causing shortness of breath?
- •
Subxiphoid long-axis view reviewing IVC distensibility and respirophasic variations
- •
- 1.
If possible, obtain at least two views to confirm findings. Patient acuity, mobility, body habitus, and time constraints are all factors that may limit the number of views that can be obtained.
Lung
- •
Pleura—Is there a pneumothorax?
- •
Sagittal view obtained in the second intercostal space
- •
Review for pleural sliding; M-mode or power Doppler may be used for assistance
- •
- •
Parenchyma
- •
Is there pulmonary edema?
- •
Sagittal view obtained in the second intercostal space
- •
Review for B-lines
- •
- •
Is there pleural effusion or consolidation?
- •
Coronal view obtained in the right and left upper quadrant flanks evaluating the pleural space above the diaphragm
- •
Review the pleural space for presence of pleural effusion or consolidation from pneumonia or masses
- •
The “spine sign” is a clue to thoracic pathology
- •
- •
An algorithmic approach has been shown to narrow differential diagnoses and increase diagnostic confidence and accuracy in the setting of dyspnea, chest pain, or symptomatic hypotension. Fig. 12.1 presents the potential views to be interrogated as part of this algorithmic approach.

Examples and Cases
The following cases exemplify the use of an ultrasound protocol such as the one we propose, as well as examples of the pathology ultrasound can help uncover.
Case 1. Pericardial Effusion with Tamponade
A 54-year-old female with history of colon cancer metastatic to the lung, on chemotherapy, presents to the emergency department for dyspnea. She describes gradually worsening nonproductive cough and shortness of breath over the past week and noted worsening abdominal swelling and abdominal pain with nausea. She denies fever. Her physical examination is notable for tachycardia, lack of fever, and distended and tender abdomen.
Differential diagnosis in this case is broad and includes worsening metastatic disease, pleural effusion, pneumonia with or without empyema, acute coronary syndrome, small bowel obstruction, and pericardial effusion. Given the vague symptoms and large differential diagnosis, ultrasound would be helpful in excluding some etiologies on the differential and help the provider reach a diagnosis.
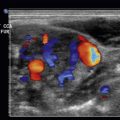
Utilizing the algorithmic approach as described earlier, the provider obtained point-of-care ultrasound (POCUS) images to aid with diagnosis. Fig. 12.2 shows the parasternal long-axis view with a large pericardial effusion and right ventricular collapse (better noted in cine-loop format, ![]() ). Ultrasound of the lungs indicated an absence of B-lines and pleural effusions ( Figs. 12.3–12.6 ), arguing against a pulmonary cause for the patient’s symptoms. The IVC is seen to be plethoric and noncollapsible, further supporting the diagnosis of tamponade ( Fig. 12.7 , better noted in cine-loop format,
). Ultrasound of the lungs indicated an absence of B-lines and pleural effusions ( Figs. 12.3–12.6 ), arguing against a pulmonary cause for the patient’s symptoms. The IVC is seen to be plethoric and noncollapsible, further supporting the diagnosis of tamponade ( Fig. 12.7 , better noted in cine-loop format, ![]() ). The patient was taken to the catheterization laboratory for a pericardial drain placement.
). The patient was taken to the catheterization laboratory for a pericardial drain placement.






Case 2. Pulmonary Embolus
A 75-year-old female presented to the emergency department with syncope. She was found to be febrile and hypotensive on arrival, and bedside laboratory testing revealed a lactic acid of 5. She had an indwelling Foley catheter placed for workup of vaginal bleeding a week prior.
Differential diagnosis included sepsis from presumed urinary tract infection.
The patient did not improve with usual sepsis care. Despite fluid resuscitation and broad-spectrum antibiotics, she remained hypotensive. Providers performed point-of-care ultrasound.
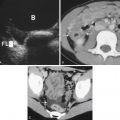
The apical four-chamber view showed right ventricular enlargement and a mobile clot through the tricuspid valve, consistent with a clot in transit ( Fig. 12.8 and ![]() ). This clot can also be seen in the right atrium in the subxiphoid view in Fig. 12.9 and
). This clot can also be seen in the right atrium in the subxiphoid view in Fig. 12.9 and ![]() . The IVC, as seen in Fig. 12.10 and
. The IVC, as seen in Fig. 12.10 and ![]() , is plethoric and noncollapsible. While undergoing computed tomography to evaluate for pulmonary embolus, the patient lost pulses and suffered a cardiac arrest. Fig. 12.11 shows a parasternal long-axis view of the heart during the code. Absence of cardiac activity can be seen better in cine-loop format in
, is plethoric and noncollapsible. While undergoing computed tomography to evaluate for pulmonary embolus, the patient lost pulses and suffered a cardiac arrest. Fig. 12.11 shows a parasternal long-axis view of the heart during the code. Absence of cardiac activity can be seen better in cine-loop format in ![]() . Lung views from the patient ( Figs. 12.12–12.15 ) show no pathology to explain the patient’s symptoms. In conclusion, the point-of-care ultrasound showed a right ventricular thrombus in transit between the right atrium and right ventricle, together with a dilated right ventricle, consistent with acute pulmonary embolus and obstructive shock physiology. The cause of the cardiac arrest was most likely the breaking off of the clot in the right atrium/ventricle. This was in contrast to the initial impression of urinary tract infection causing sepsis.
. Lung views from the patient ( Figs. 12.12–12.15 ) show no pathology to explain the patient’s symptoms. In conclusion, the point-of-care ultrasound showed a right ventricular thrombus in transit between the right atrium and right ventricle, together with a dilated right ventricle, consistent with acute pulmonary embolus and obstructive shock physiology. The cause of the cardiac arrest was most likely the breaking off of the clot in the right atrium/ventricle. This was in contrast to the initial impression of urinary tract infection causing sepsis.








Case 3. Pneumonia
A 40-year-old female with nonsquamous cell lung cancer, known right lung necrotic mass, currently on chemotherapy, presented to the emergency department with worsening dyspnea, nonproductive cough, and subjective fevers for 2 days but worse since her bronchoscopy on the morning of the day of presentation. On examination, she was noted to be hypoxic to 90% on room air, hypotensive to 90/50 mm Hg, with diminished right-sided breath sounds.
The differential diagnosis in this case is broad, including pneumonia, pulmonary embolus, worsening tumor burden, pleural effusion, and empyema.
Using the algorithm described earlier, the providers were able to narrow this differential diagnosis. A parasternal long-axis view, as seen in Fig. 12.16 , demonstrated no pericardial effusion, normal ejection fraction (better evaluated in cine-loop format, ![]() ), no right ventricular enlargement to suggest right heart strain, and no aortic root dilation. A short-axis view at the level of the papillary muscles also demonstrated normal ejection fraction ( Fig. 12.17 and
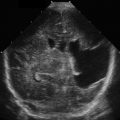
), no right ventricular enlargement to suggest right heart strain, and no aortic root dilation. A short-axis view at the level of the papillary muscles also demonstrated normal ejection fraction ( Fig. 12.17 and ![]() ). The patient had no evidence of alveolar interstitial syndrome in anterior lung fields ( Figs. 12.18 and 12.19 ). The right lung base did show a spine sign, hepatization of lung, and air bronchograms, consistent with a unilateral infiltrative process ( Figs. 12.20 and 12.21 ,
). The patient had no evidence of alveolar interstitial syndrome in anterior lung fields ( Figs. 12.18 and 12.19 ). The right lung base did show a spine sign, hepatization of lung, and air bronchograms, consistent with a unilateral infiltrative process ( Figs. 12.20 and 12.21 , ![]() ). The diameter of the IVC was small, and the IVC was fully collapsible, further supporting an infectious cause of the symptoms ( Fig. 12.22 ,
). The diameter of the IVC was small, and the IVC was fully collapsible, further supporting an infectious cause of the symptoms ( Fig. 12.22 , ![]() ). In this case, point-of-care ultrasound revealed the diagnosis of a right-sided pneumonia causing the patient’s symptoms, as evidenced by the right-sided consolidation with air bronchograms and absence of other pathologies.
). In this case, point-of-care ultrasound revealed the diagnosis of a right-sided pneumonia causing the patient’s symptoms, as evidenced by the right-sided consolidation with air bronchograms and absence of other pathologies.