Arrhythmogenic right ventricular cardiomyopathy (ARVC) is a heritable heart muscle disease characterized by fibrofatty replacement of, predominantly, the right ventricular (RV) myocardium, which predisposes patients to potentially life-threatening arrhythmias and ventricular dysfunction. Affected patients typically present between the second and fourth decade of life with arrhythmias coming from the RV. ARVC is an unusual condition, with an estimated prevalence of 1 in 1000 to 1 in 5000 Caucasian individuals. Approximately 60% of index cases harbor mutations in genes encoding the cardiac desmosome, a structure that provides mechanical connection between cardiomyocytes. The defective mechanical connections in ARVC may structurally be represented by global or regional contraction abnormalities, ventricular enlargement, and/or fibrofatty replacement. As such, accurate evaluation of cardiac morphology and function is essential for ARVC diagnosis and management.
ARVC constitutes a diagnostic challenge for the cardiovascular magnetic resonance (CMR) physician. The variation in shape and contraction patterns of the normal RV complicates identification of early, subtle RV disease. In addition, many imaging centers have little experience with evaluating ARVC, and gaining experience is difficult because of the low prevalence of disease. Missing a diagnosis of ARVC may prove to be fatal, whereas overdiagnosis may lead to therapeutic repercussions in essentially healthy individuals. Despite these challenges, CMR is generally acknowledged to be the “best” noninvasive test for ARVC diagnosis and to distinguish ARVC from other cardiomyopathies. Thus the aim of this chapter is to review current knowledge of ARVC that is useful for CMR interpretation of individuals suspected of this disease. Our emphasis will be on ARVC diagnosis and management, including diagnostic criteria, CMR acquisition/analysis, and common regional morphologic and functional abnormalities in ARVC.
A Historical Perspective on Arrhythmogenic Right Ventricular Cardiomyopathy
The first comprehensive clinical description of ARVC dates back to 1982, when Marcus and colleagues described a cohort of 24 patients with arrhythmias originating from the RV and (right) heart failure. Using echocardiography, angiography, and histologic specimens obtained at surgery, they described ARVC as a regional RV disease with paper-thin RV myocardium, myocardial cell loss, and abundant subepicardial fat. The abnormal RV regions were traditionally thought to be located to the so-called triangle of dysplasia (i.e., RV inflow tract, RV apex, and RV outflow tract [RVOT]). In subsequent years, several ARVC manuscripts have focused on gross or histologic evidence of myocardial cell loss with fibrofatty replacement. It is important to note that these observations were made in tertiary centers, without the advantage of genetic testing and without sensitive diagnostic criteria. Over the last decade, several collaborative efforts in the United States and Europe have significantly enhanced our understanding of (the role of CMR in) ARVC diagnosis, clinical characteristics, and management. These advancements are reviewed here.
Diagnosis of Arrhythmogenic Right Ventricular Cardiomyopathy
Diagnosis of ARVC is challenging because no single modality is sufficiently specific to establish an ARVC diagnosis. Therefore multiple sources of diagnostic information are combined in a complex set of diagnostic criteria. Definite ARVC diagnosis is based on the presence of major and minor criteria encompassing structural, histologic, electrocardiographic, arrhythmic, and family history criteria ( Box 34.1 ). These “Task Force” criteria (TFC) were first established in 1994 and updated in 2010 to improve (1) the specificity of the TFC by including quantitative metrics for diagnosis; and (2) sensitivity of the TFC in individuals who have a high likelihood of inherited/genetic disease. Structural criteria for ARVC diagnosis may be based on echocardiography, angiography, or CMR. However, echocardiographic evaluation of the RV is difficult because of its complex geometry, and a recent study has shown that echocardiographic evaluation of subtle RV changes may be unreliable. In addition, angiography is invasive and should not be employed as a first-line screening test for ARVC. In contrast, CMR allows for noninvasive multiplane morphologic and functional evaluation, as well as tissue characterization in a single investigation, and has emerged as the imaging modality of choice in ARVC.
Global or Regional Dysfunction and Structural Alterations
Major
Two-Dimensional Echocardiography Criteria
Regional RV akinesia, dyskinesia, or aneurysm and one of the following measured at end diastole:
- •
PLAX RVOT ≥32 mm (PLAX/BSA ≥19 mm/m 2 ), or
- •
PSAX RVOT ≥36 mm (PSAX/BSA ≥21 mm/m 2 ), or
- •
Fractional area change ≤33%
- •
Cardiovascular Magnetic Resonance Criteria
Regional RV akinesia or dyskinesia or dyssynchronous RV contraction and one of the following:
- •
RV EDV/BSA ≥110 mL/m 2 (male) or ≥100 mL/m 2 (female), or
- •
RV ejection fraction ≤40%
- •
Right Ventricular Angiography Criteria
Regional RV akinesia, dyskinesia, or aneurysm
Minor
Two-Dimensional Echocardiography Criteria
Regional RV akinesia or dyskinesia or dyssynchronous RV contraction and one of the following measured at end diastole:
- •
PLAX RVOT ≥29 to <32 mm (PLAX/BSA ≥16 to <19 mm/m 2 ), or
- •
PSAX RVOT ≥32 to <36 mm (PSAX/BSA ≥18 to <21 mm/m 2 ), or
- •
Fractional area change >33% ≤40%
- •
Cardiovascular Magnetic Resonance Criteria
Regional RV akinesia or dyskinesia or dyssynchronous RV contraction and one of the following:
- •
RV EDV/BSA ≥100 to 110 mL/m 2 (male) or ≥90 to 100 mL/m 2 (female)
- •
RV ejection fraction >40 to ≤45%
- •
Tissue Characterization of Wall
Major
Residual myocytes <60% by morphometric analysis (or <50% if estimated), with fibrous replacement of the RV free wall myocardium in ≥1 sample, with or without fatty replacement of tissue on endomyocardial biopsy
Minor
Residual myocytes 60% to 75% by morphometric analysis (or 50% to 65% if estimated), with fibrous replacement of the RV free wall myocardium in ≥1 sample with or without fatty replacement of tissue on endomyocardial biopsy
Repolarization Abnormalities
Major
Inverted T waves in right precordial leads (V1, V2, and V3) or beyond in individuals >14 years of age (in the absence of complete RBBB)
Minor
Inverted T waves in V1 and V2 in individuals >14 years of age (in the absence of complete RBBB) or in V4, V5, and V6
Inverted T waves in leads V1, V2, V3, and V4 in individuals >14 years of age in the presence of complete RBBB
Depolarization/Conduction Abnormalities
Major
Epsilon wave (reproducible low-amplitude signals between end of QRS complex to onset of T wave) in the right precordial leads (V1, V2, or V3)
Minor
Late potentials by SAECG in ≥1 of 3 parameters in the absence of a QRS duration of ≥110 ms on standard ECG:
- •
Filtered QRS duration (fQRS) ≥114 ms
- •
Duration of terminal QRS <40 µV ≥38 ms
- •
Root-mean-square voltage of terminal 40 ms ≤20 µV
- •
Terminal activation duration ≥55 ms measured from the nadir of the end of all depolarization deflections, including R′, in V1, V2, or V3 in absence of complete RBBB
Arrhythmias
Major
Nonsustained or sustained VT of LBBB morphology with superior axis
Minor
Nonsustained or sustained VT of RVOT configuration, LBBB morphology with inferior axis or of unknown axis
>500 PVCs per 24 hours on Holter monitoring
Family History
Major
ARVC in first-degree relative who meets Task Force Criteria
ARVC confirmed pathologically at autopsy or surgery in first-degree relative
Identification of pathogenic mutation categorized as associated or probably associated with ARVC in the patient under evaluation
Minor
History of ARVC in first-degree relative in whom it is not possible to determine whether the family member meets Task Force Criteria
Premature sudden death (<35 years of age) due to suspected ARVC in first-degree relative
ARVC confirmed pathologically or by current Task Force Criteria in second-degree relative
ARVC, Arrhythmogenic right ventricular cardiomyopathy; BSA, body surface area; ECG, electrocardiogram; EDV, end-diastolic volume; LBBB, left bundle branch block; PLAX, parasternal long axis; PSAX, parasternal short axis; PVCs, premature ventricular complexes; RBBB, right bundle branch block; RV, right ventricular; RVOT, right ventricular outflow tract; SAECG, signal-averaged electrocardiogram; VT, ventricular tachycardia.
Cardiovascular Magnetic Resonance Task Force Criteria and Their Derivation
The diagnostic TFC for CMR require the presence of both qualitative findings (RV regional akinesia, dyskinesia, dyssynchronous contraction) and quantitative metrics (decreased ejection fraction or increased indexed RV end-diastolic volume) (see Box 34.1 ). Quantitative values for RV volume and function for TFC were derived from a comparison of ARVC probands with normal healthy volunteers that were included in the Multi-Ethnic Study of Atherosclerosis (MESA). To ascertain cutoff values, RV dimension and function from 462 normal MESA participants were compared with 44 probands in the North American ARVC registry. Major criteria (RV ejection fraction ≤40% or indexed RV end-diastolic volume ≥110 mL/m 2 for men and ≥100 mL/m 2 for women) were chosen to achieve approximately 95% specificity. Cutoffs with high specificity invariably result in lower sensitivity; this accounts for the major CMR criteria sensitivity of 68% to 76%. Minor criteria (RV ejection fraction 40%–45% or indexed RV end-diastolic volume 100–110 mL/m 2 for men and 90–100 mL/m 2 for women) had a higher sensitivity (79%–89%), but a consequently lower specificity (85%–97%).
Quantitative Evaluation of the Right Ventricle in the Revised Task Force Criteria
Several studies have shown that including quantitative metrics as a component to the CMR TFC increased its diagnostic value, mainly through an increase in positive predictive value. However, the CMR physician should be aware of its limitations. First, although quantitative measures reduce subjectivity of CMR TFC, there is substantial inter-reader variability for the RV. In our experience, two physician readers had excellent agreement (within ~5% of reference values) only after training on approximately 100 CMR cases. In clinical practice, we expect it would be difficult to achieve reproducibility of less than 10% for RV parameters. In addition, cutoffs for ARVC criteria were derived from the MESA study that used the fast gradient recalled echo (FGRE) technique, whereas a majority of study subjects in the US ARVC study had steady-state free precession (SSFP) cine images. RV volumes by SSFP are at least 10% larger than those measured with the FGRE technique. SSFP images provide superior contrast between blood and endocardium at the endocardial border, with less blood flow dependence. Also, the revised TFC used MESA subjects whose mean age was 60 years. ARVC subjects in the Task Force study were 20 to 30 years younger on average. Since that time, Chahal et al. determined that, among MESA participants aged 45 to 84 years old, RV end-diastolic volume decreased 4.6% per decade. A very similar percentage of approximately 4% decrease per decade was obtained by Maceira et al. using the SSFP technique in subjects aged 20 to 80 years old. Adjusting for body surface area did not remove the dependence of RV volume on age.
Fortuitously, the issues of pulse sequence and older reference population in the MESA study approximately balance each other. As an example, the CMR cutoff values for RV size in ARVC (see Box 34.1 ) are ≥110 mL/m 2 (male) or ≥100 mL/m 2 (female) for major criteria. These values closely correspond to the 95th percentile confidence limits of RV volumes for normal subjects less than 60 years of age. Thus until further studies are available, we feel that the current RV metrics in the revised TFC remain highly relevant. Important further developments are necessary to improve the reproducibility of RV quantification by CMR. When evaluating younger patients, CMR physicians should keep in mind that the size of the RV is expected to be ~10% larger in a 20-year-old patient compared with a 40-year-old patient.
Cardiovascular Magnetic Resonance Acquisition for Suspected Arrhythmogenic Right Ventricular Cardiomyopathy
The CMR protocol that we recommend for evaluation of patients with possible ARVC is shown in Table 34.1 . The protocol has been designed to evaluate the RV for abnormalities in structure and tissue characterization while enabling quantitative evaluation. For black-blood imaging, fast spin echo, or turbo spin echo imaging sequences are ideal. The RV free wall and RVOT are best evaluated in the axial black-blood images. The stack of axial images should include the entire RV. This can be accomplished in 6 to 8 slices at intervals (slice thickness + gap) of about 1 cm. Most of the diagnostic information is obtained in the slices centered on the middle of the RV. Obtaining an excessive number of image slices will adversely prolong the examination.
| Sequence | Imaging Plane | Parameters | Comments |
|---|---|---|---|
Double inversion recovery TSE/FSE
|
| TR = 2 RR intervals, TE = 5 ms (minimum-full) (GE), TE = 30 ms (Siemens), slice thickness = 5 mm, interslice gap = 5 mm, and FOV = 28–34 cm ETL = 16–24 | This sequence provides optimal tissue characterization of the RV free wall. Prescribe from the pulmonary artery to the diaphragm. Fat suppression improves reader confidence in diagnosis of RV fat infiltration. |
| SSFP bright-blood cine images | Axial, four-chamber, and short axis. RV three-chamber (optional) | TR/TE minimum, flip angle = 45–70 degrees, slice thickness = 8 mm, interslice gap = 2 mm FOV = 36–40 cm, 16–20 views per segment. Parallel imaging N = 2 is desirable | Axial images are best to assess RV wall motion. RV quantitative analysis is performed on the short-axis cine images. |
| Gadolinium is administered according to institutional protocol (usually 0.15–0.2 mmol/kg). | |||
| TI scout | Four-chamber | TI scout sequences or trial TI times to suppress normal myocardium for the right inversion time | |
| Delayed gadolinium imaging (fast gradient echo technique with PSIR recommended) | Axial, short axis, four-chamber, and vertical long axis | TR/TE per manufacturer recommendations flip angle = 20–25 degrees, slice thickness = 8 mm, interslice gap = 2 mm FOV = 36–40 cm. No parallel imaging. Use PSIR, if available. | PSIR is more robust and independent of TI time. Optimal for imaging fibrosis. LV epicardial enhancement in the inferolateral wall has been reported in classic ARVC and in left dominant forms. |
For cine imaging, SSFP imaging is preferred at 1.5 T. Insufficient information at 3 T is available to determine if SSFP or FGRE is superior. Quantitative analysis of the RV and left ventricle (LV) is performed on short-axis images. Thus 10 to 12 slices encompassing the entire ventricular volume must be obtained. We prescribe these images beginning approximately 1 cm above the valve plane and increment toward the apex of the ventricles. Cine images should also be obtained in standard long-axis views of the LV. Some sites prefer to also acquire a vertical long-axis view of the RV. Finally, we routinely obtain a stack of transaxial images of the RV at the same slice positions as the black-blood images described above. Given modern CMR scanners, the temporal resolution of cine images is typically around 40 ms.
Delayed gadolinium images are best obtained using phase-selective inversion recovery (PSIR), a sequence which does not depend upon identifying the precise inversion time (TI). In patients with significant ventricular ectopy, a low dose of a beta-blocker (metoprolol 25–50 mg) is recommended for arrhythmia suppression during the CMR scan.
Cardiovascular Magnetic Resonance Findings Associated With Arrhythmogenic Right Ventricular Cardiomyopathy
Use of CMR in ARVC was first described in 1987, when Casolo et al. demonstrated intramyocardial fat deposits using spin echo sequences in an ARVC patient. Two years later, Wolf and colleagues described “dysplastic lesions in the RV wall presenting as fat-like high signals” in 7 ARVC patients. Over the years that followed, CMR findings associated with ARVC include RV wall thinning, RVOT enlargement, trabecular disarray, fibrofatty replacement, ventricular dilatation, and global systolic dysfunction. However, our current emphasis is on regional wall motion abnormalities as the earliest and most reliable abnormalities identified by CMR.
Regional Systolic Wall Motion Abnormalities
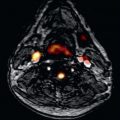
Most of our information about structural abnormalities in ARVC comes from studies in subjects with a predominant RV phenotype. Besides global reduction in RV function, more subtle regional disease of the RV has been described in the literature using a variety of terms, including focal bulges, microaneurysms, segmental dilatation, and regional hypokinesis. In the current TFC, the terms “akinesia” (lack of motion) and “dyskinesia” (abnormal movement, instead of contracting in systole, the myocardium bulges outward in systole), and “dyssynchronous” (regional peak contraction occurring at different times in adjacent myocardium) are used for all imaging modalities (CMR, echocardiography, and angiography) to describe regional wall motion abnormalities in ARVC. Characteristic examples of RV wall motion abnormalities are shown in Fig. 34.1 . Microaneurysms are not explicitly described in the revised TFC; overuse of this term was considered by the Task Force members to be misinterpreted by CMR physicians, resulting in false-positive diagnoses. However, microaneurysms are characterized by regional akinesia/dyskinesia in the revised criteria.

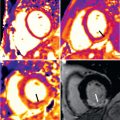
The location of regional wall motion abnormalities of the RV was not addressed in the revised TFC. We now recognize that the distal RV (from the moderator band to the apex in long-axis views) shows highly variable contraction patterns in the normal individual. In addition, a recent study has shown that the RV apex is spared in early ARVC. Therefore we emphasize the significance of regional wall motion abnormalities in the subtricuspid region in ARVC. An excellent example of this is the “accordion sign” that represents a focal “crinkling” of the myocardium ( Fig. 34.2 ). In terms of TFC, the accordion sign is caused by a small region of highly localized myocardium with dyssynchronous contraction.

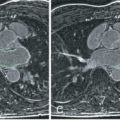
Late Gadolinium Enhancement
Myocardial late gadolinium enhancement (LGE) is a well-validated technique for assessment of myocardial fibrosis, and has frequently been associated with ARVC: RV LGE has been observed in up to 88% of ARVC patients, whereas LV LGE was reported in up to 61% of cases. A characteristic example is shown in Fig. 34.3 . Given that one of the pathologic hallmarks of ARVC is fibrofatty replacement of the myocardium, it is important to note that LGE is not incorporated in the current diagnostic TFC. Although the Task Force did recognize the presence of LGE in many patients with ARVC, several limitations withheld its inclusion in the TFC. First, detection of LGE in the RV is greatly hampered by the thin RV wall. In ARVC, RV wall thinning is pronounced, which makes the LGE technique less reliable than for the LV. Second, distinguishing fat from fibrosis by LGE sequences is challenging, which makes its interpretation highly subject to the CMR physician’s experience. Last, LV LGE is nonspecific and has a wide differential diagnosis. LGE may be observed in many mimics of ARVC, such as sarcoidosis, myocarditis, amyloidosis, and dilated cardiomyopathy (DCM).