34
Arteriography and Venous Sampling of the Parathyroid Glands, Pancreas, and Adrenal Glands
All endocrine localization procedures, including arteriography and venous sampling, are used in patients with clinically evident syndromes of hormone excess. There are two indications for localization procedures: localization of otherwise occult tumors and differentiation between single and multiple gland involvement. Clinical syndromes and the invasive localization procedures that may be indicated in their workup are listed in Table 34-1. These procedures may be required for tumor localization in patients with any of these disorders. Differentiation of single and multiple gland involvement is a common problem in patients with Conn syndrome (hyperaldosteronism) and a rare problem in patients with virilizing tumors.
None of the endocrine diseases listed in Table 34-1 are common, and most are rare. It follows that the localization procedures listed in Table 34-1 also are rarely used. Except in large academic medical centers, most of these procedures are unlikely to be performed on a sufficiently regular basis that a resident or fellow in interventional radiology will become familiar with them. Nonetheless, it is useful to know when, why, and how they are performed, as well as how they are interpreted. This chapter describes the catheter-based localization procedures used for endocrine disorders of the parathyroid glands, the pancreas, and the adrenal glands. Nowadays, localization procedures are virtually never required for patients with pheochromocytoma and are needed so rarely in patients with virilizing tumors that they are not discussed here, but they are reviewed elsewhere.1
Arteriography and venous sampling are not used as first-line techniques in patients with endocrine disorders because of their greater risk and expense compared with noninvasive radiologic techniques. The specific order in which radiologic procedures are used varies for each organ and disease. Appropriate protocols are briefly described below in sections devoted to specific organs.
 General Principles of Endocrine Localization
General Principles of Endocrine Localization
Endocrine localization procedures differ from most other radiologic studies in that their purpose is not to establish a diagnosis but rather to identify an adenoma, carcinoma, or hyperplastic gland whose existence has already been established by endocrine testing but whose location is unknown. The diagnosis of a disorder of hormone excess never requires demonstration of a tumor or enlarged gland. The most basic principle of endocrine localization efforts is “diagnosis first, localization second.” These studies are certain to yield misleading results if the diagnosis is wrong.
Equally important is an understanding of the advantages and limitations of venous sampling procedures. These are physiologic studies, not imaging procedures. They do not require visualization of an abnormality to provide localization. This is both the great strength and the major weakness of this technique.
On the one hand, because it is not necessary to visualize a tumor, the sensitivity of venous sampling is independent of tumor size. It is equally good for the detection of 1-mm and 3-cm lesions. The only requirements are that the lesion secretes sufficient hormone to be detected and that the appropriate vein is sampled. Nonfunctioning adenomas and similar red herrings do not cause false-positive results with venous sampling, as they may with imaging studies.
| Organ | Localization procedure |
| Pituitary | |
| Cushing syndrome/disease | Petrosal sinus sampling |
| Ectopic ACTH syndrome | |
| Parathyroid | |
| Hyperparathyroidism | Parathyroid arteriography |
| Parathyroid venous sampling | |
| Pancreas | |
| Zollinger-Ellison syndrome | Pancreatic arteriography |
| Insulinoma | Arterial stimulation and venous sampling |
| Adrenal | |
| Conn syndrome | Adrenal venous sampling |
| Ovary | |
| Virilizing tumors | Adrenal and ovarian venous sampling |
| ACTH, adrenocorticotropic hormone. |
The disadvantage of the procedure is that, because the tumor is never seen during a venous sampling procedure, it cannot be localized precisely. Instead, venous sampling permits regionalization; that is, it defines a territory drained by a specific vein or veins in which the abnormality must lie. In addition, because tumor presence is presumed based on the evidence of hormone production, the test is only accurate if all production of the hormone by normal glandular tissue is suppressed. When venous sampling procedures are performed on patients without endocrine disease, false-positive studies are virtually certain. For this reason, as already stated, a diagnosis of endocrine hyperfunction must be established before localization procedures are begun.
 Hyperparathyroidism
Hyperparathyroidism
Clinical review
In normal subjects, elevated serum calcium inhibits production of parathyroid hormone (PTH) through a negative feedback loop.Primary hyperparathyroidism is defined as excess production of PTH resulting from a loss of the normal feedback inhibition from elevated serum calcium. Increased hormone levels may result from parathyroid hyperplasia (multiple gland involvement), parathyroid adenoma (involvement of a single gland), or, rarely, parathyroid carcinoma. The elevated serum calcium concentration is caused by increased renal retention, bone resorption, and increased absorption from the gut. Patients may develop osteolysis leading to bone pain and pathologic fractures, urinary stone disease, and neuromuscular physiologic dysfunction. Symptoms may be subtle, vague, and nonspecific. Increasingly, the diagnosis of abnormal parathyroid function is made through the widespread use of routine laboratory screening panels, which include calcium assays coupled with improved techniques for measurement of PTH.2,3
Localization
Most experienced endocrine surgeons believe that noninvasive imaging studies are not required in patients who present with primary hyperparathyroidism, because the initial neck exploration is curative in more than 90% of these patients.4–7 No preoperative localization studies are necessary in these patients,8,12 although some surgeons advocate their use in combination with unilateral neck exploration.13–17 There is general agreement that patients with persistent or recurrent hypercalcemia following surgery will benefit from radiologic localization prior to reoperation, because each additional surgical exploration is more difficult, carries a alreadyhigher risk of vocal cord paralysis, and is less likely to succeed.9
An effective localization protocol for these patients has been devised.9 Noninvasive studies should be performed first, because they are less expensive and less risky. The imaging studies with the greatest sensitivity at present are sestamibi scintigraphy, computed tomography (CT), magnetic resonance imaging (MRI), and ultrasound. These modalities are all operator dependent in terms of performance, interpretation, or both; their sensitivity and false-positive rates vary widely and overlap. Sestamibi scintigraphy is probably the single best noninvasive test.18 We believe an abnormal parathyroid gland should be demonstrated by at least two of these modalities before localization is considered complete in order to avoid false-positive localizations. The order in which the various studies are performed should be based on individual institutional experience. All imaging protocols should account for the possibility of ectopic mediastinal or suprathyroid parathyroid glands, although most parathyroid glands identified at reoperation are in the neck.
At most institutions, if noninvasive studies are negative or equivocal, parathyroid arteriography is performed next.9,19 Parathyroid arteriography will demonstrate an abnormal parathyroid gland in approximately 60% of patients in whom noninvasive studies are negative or equivocal. Parathyroid venous sampling, the final localization procedure in the workup of these patients, will permit localization to one side of the neck or to the mediastinum in 80 to 90% of the remaining few patients in whom all other studies have failed to image an abnormal gland.
Anatomy
Most people have four parathyroid glands, although as few as three and as many as eight have been reported.20 In the normal anatomic position, the glands adhere to the posterior surface of the thyroid gland (Fig. 34-1). They also may be ectopic and may be located anywhere from the angle of the jaw to the mediastinum (Fig. 34-2). Knowledge of the common locations of normal and ectopic glands in a prerequisite to successful localization by interventional techniques. Fortunately, most ectopic glands lie along specific, well-defined pathways of embryologic descent, either in the anterior mediastinum and thymus or in the tracheoesophageal groove and superior mediastinum.20,21
Each parathyroid gland usually is supplied by a single artery.22,23 The arterial supply to parathyroid glands located in the normal anatomic position is provided by the paired superior thyroid arteries from the external carotid artery and the paired inferior thyroid arteries from the thyrocervical trunk (Fig. 34-1). Anterior mediastinal and thymic glands are supplied by branches of the internal mammary artery (Fig. 34-3). Superior mediastinal and tracheo-esophageal groove glands usually are supplied by a branch of the inferior thyroid artery. The venous anatomy of the thyroid bed is quite variable. Detailed descriptions of the venous anatomy relevant to parathyroid venous sampling have been published24–26 and should be reviewed by the angiographer prior to attempting this study.
Venous drainage from the parathyroid glands commonly occurs through ipsilateral superior, middle, and inferior thyroid veins (Figs. 34-4 and 34-5). The superior thyroid vein drains through the facial vein into the internal jugular vein, and the middle thyroid vein drains directly into the internal jugular vein. Both inferior thyroid veins commonly drain into the left brachiocephalic vein, either separately or by a combined trunk. Less commonly, the right inferior thyroid vein drains directly into the right brachiocephalic vein (see Fig. 34-4). The left thymic vein empties into the left innominate vein; elevations of PTH in the thymic vein suggest that the abnormal parathyroid gland is located in the anterior mediastinum. The anatomy and anastomotic connections of this vein are quite variable, and mediastinal parathyroid glands often drain into the inferior thyroid veins (Fig. 34-6).27 Analysis of the venous drainage patterns observed at arteriography is essential to permit correct interpretation of the results of venous sampling.
Arteriography
Digitally subtracted, superselective, six-vessel studies (bilateral thyrocervical trunks, internal mammary arteries, and common carotid arteries) deliver the highest yield.28 Venous-phase images are also obtained to provide “roadmaps” and to define venous drainage patterns in cases where venous sampling proves necessary (see Fig. 34-5). If the common carotid artery injections are not clearly negative, bilateral superior thyroid artery injections may be helpful to clarify the arteriographic findings. If all else fails, an arch aortogram may reveal a thyroidea ima artery (present in approximately 6% of persons),29 which most commonly arises from the aortic arch, the innominate artery, or the right common carotid artery and supplies variable portions of the thyroid and parathyroid glands.29,30 This regimen demonstrates an abnormal parathyroid gland in approximately 60% of the patients in whom noninvasive imaging is negative or inconclusive.19,31 Parathyroid arteriography has a low false-positive rate.
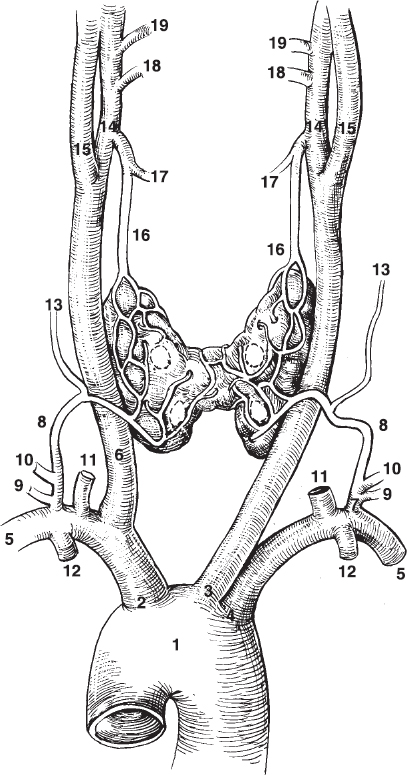
FIGURE 34-1. Arterial supply to the thyroid and parathyroid glands. The parathyroid glands are adherent to the posterior surface of the thyroid gland. The superior and inferior thyroid arteries ramify along the posterior aspect of the thyroid gland, but are shown here on the anterior surface for the purpose of clarity. The drawing displays the typical supply from the inferior and superior thyroid arteries. When ectopic parathyroid glands are located in the thymus of elsewhere in the anterior mediastinum, they are usually supplied by a branch of the internal mammary artery (see Fig. 34-3). 1, aorta; 2, brachiocephalic trunk; 3, left common carotid; 4, left subclavian; 5, subclavian; 6, right common carotid; 7, thyrocervical trunk; 8, inferior thyroid; 9, suprascapular; 10, tranverse cervical; 11, vertebral; 12, internal thoracic; 13, ascending cervical; 14, external carotid; 15, internal carotid; 16, superior thyroid; 17, superior laryngeal; 18, lingual; 19, facial.
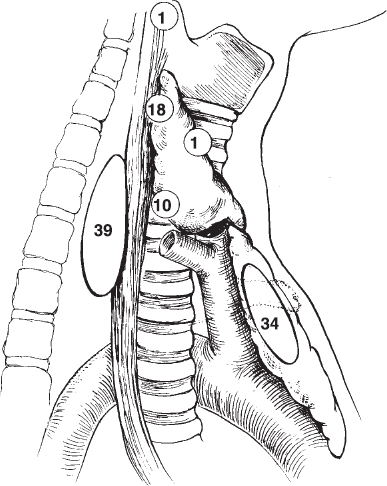
FIGURE 34-2. Location of ectopic parathyroid glands based on a series of 104 glands identified at reoperation. The numbers are percentages. Note the frequency of tracheoesophageal and thymic glands. These represent, respectively, ectopic superior and inferior glands. Tracheoesophageal groove glands typically are supplied by the inferior thyroid artery, whereas thymic glands are supplied by the internal mammary artery. (Modified from Wang C-A. Parathyroid re-exploration: a clinical and pathological study of 112 cases. Ann Surg1977;186:140–145, with permission.)
The aortic arch, common carotid arteries, and, if necessary, the superior thyroid arteries may be catheterized by using standard techniques and catheters; however, these are the least useful vessels for parathyroid localization. Catheterization of the thyrocervical trunk, a critical vessel in parathyroid arteriography, is often more difficult. It may be helpful to steam a Berenstein-type catheter so that the curvature of the bend is more gradual and the angle is tighter. The internal mammary artery, which arises anteriorly or anteroinferiorly from the subclavian artery, and not directly inferiorly, should be catheterized first. After internal mammary arteriography is performed, the catheter is withdrawn slowly until it is near the orifice of the internal mammary artery. As gentle puffs of contrast material are injected, the catheter is withdrawn further and rotated anteriorly to engage the orifice of the thyrocervical trunk. The thyrocervical trunk arises from the anterior or antero-superior aspect of the subclavian artery, but it or one or more of its branches may share a common origin with the internal mammary artery.
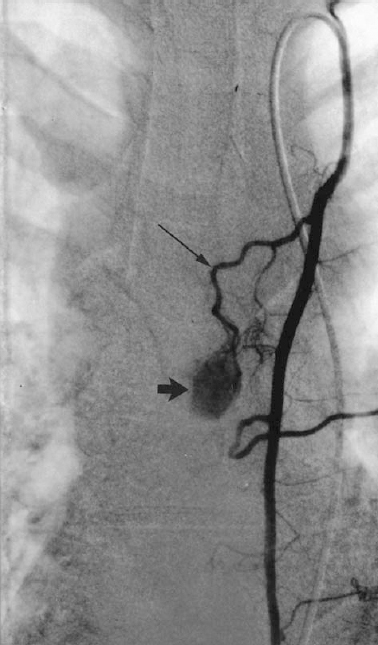
FIGURE 34-3. Parathyroid adenoma supplied by the left internal mammary artery. A single vessel from the thymic branch (long arrow) of the internal mammary artery supplies an oval, smooth-margined area of intense, homogeneous blush (wide arrow), which is typical of a parathyroid adenoma. (From Miller DL. Endocrine angiography and venous sampling. Radiol Clin North Am1993;31:1051–1067, with permission.)
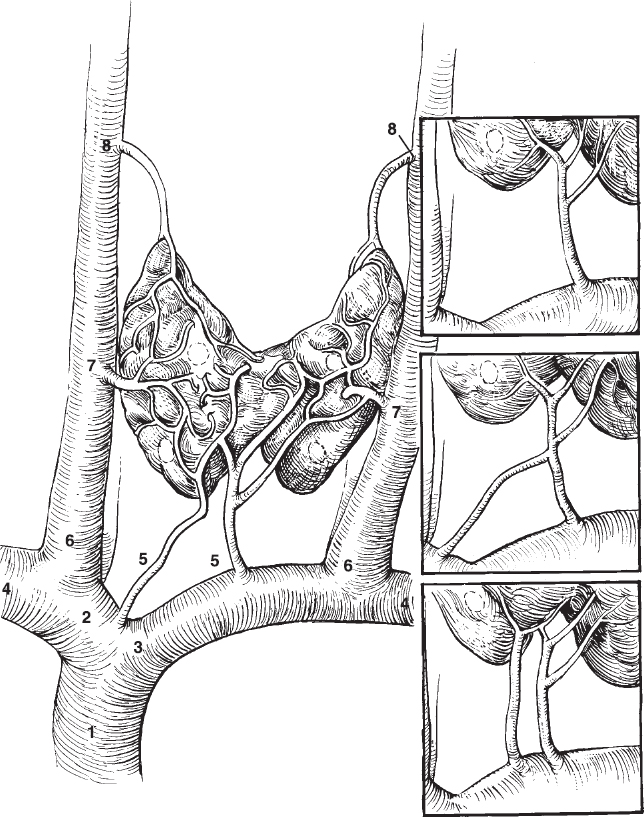
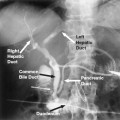
FIGURE 34-4. Anatomy of veins draining the thyroid bed and portions of the anterior mediastinum. The inferior thyroid veins, in particular, are subject to considerable variation, and both often drain into the left brachiocephalic vein, frequently as a combined trunk. The vertebral veins and thymic veins are not shown, for clarity. The vertebral veins empty in the brachiocephalic veins slightly posteromedial to the internal jugular veins and commonly have valves near their bases. The left thymic vein drains into the anteroinferior aspect of the left brachiocephalic vein in the midline. The right thymic vein drains directly into the inferior vena cava and cannot normally be catheterized. 1, superior vena cava; 2, right brachiocephalic; 3, left brachiocephalic; 4, subclavian; 5, inferior thyroid; 6, internal jugular; 7, middle thyroid; 8, superior thyroid.
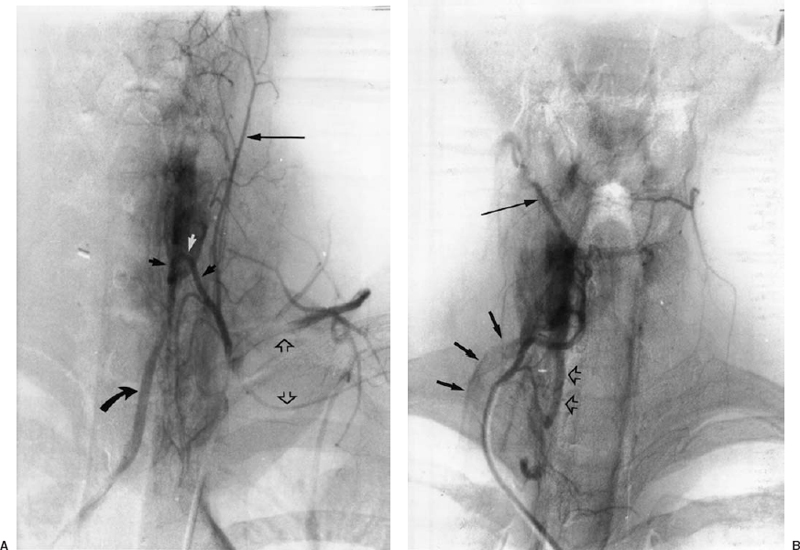
FIGURE 34-5. Thyrocervical arteriograms demonstrate arterial and venous anatomy in a patient with recurrent hyperparathyroidism following surgery. No abnormal parathyroid gland was identified on this study. A: A left thyrocervical trunk injection demonstrates the ascending cervical artery (long arrow) , the transverse cervical and suprascapular arteries (open arrows), and the typical looping course of the inferior thyroid artery (short arrows). The inferiorly directed branches of the inferior thyroid artery supply portions of the esophagus and trachea. Note the excellent demonstration of the venous drainage of the left thyroid lobe into the left inferior thyroid vein (curved arrow). B: Selective injection into the right inferior thyroid artery in the same patient demonstrates venous drainage of the right thyroid lobe into the right superior thyroid vein (long arrow), the right inferior thyroid vein (open arrows), and the right vertebral vein (short arrows). Both arteriograms also demonstrate the normal thyroid blush.
The thyrocervical trunk can be recognized by the characteristic looping course of the inferior thyroid artery (see Figs. 34-1 and 34-5) Although the inferior thyroid artery occasionally may have been ligated at the initial surgery, failure to identify this vessel usually means that the catheter is in the costocervical trunk, not the thyrocervical trunk. The costocervical trunk arises from the posterosuperior aspect of the subclavian artery, adjacent to the thyrocervical trunk, and usually is much easier to catheterize than the thyrocervical trunk. Intentional injection of the costocervical trunk should be avoided, however, because it often supplies a major feeder to the cervical spinal cord.
At arteriography, hyperplastic and adenomatous parathyroid glands are oval or rounded areas of diffuse enhancement, often superimposed on thyroid tissue (see Fig. 34-3). With optimal radiographic technique and collimation, pathologic glands as small as 4 to 5 mm may be visible. Normal parathyroid glands are never visualized angiographically in these patients because their PTH production is suppressed and the glands become quite small (1–2 mm).32
Venous sampling
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree