- •
ML and DL techniques are becomingly increasingly sophisticated. AI-based algorithms can be used to improve the accuracy of image processing, segmentation, and quantification and also allow for more robust automation.
- •
AI can be used to improve image quality or reduce radiation exposure for patient.
- •
ML and DL algorithms have demonstrated improvements in diagnostic accuracy compared with expert interpretation and current quantitative methods.
- •
AI-based techniques represent an optimal approach to integrating the vast amount of clinical, stress testing, and imaging data available with each nuclear cardiology scan.
- •
When testing the performance of AI algorithms, it is essential that test data are always kept separate from the training data to provide realistic estimates of performance.
- •
External evaluation in data from unseen centers, not just hold-out data from the same center, will provide the most realistic estimate of performance during the model deployment.
- •
For clinical translation, the ability to explain the presented results is crucial to ensuring physician acceptance. Explanation of the AI findings for a specific patient will be key to acceptance of the new AI tools by the physicians.
- •
Both DL and traditional ML methods can highlight image or clinical features, which contributes to the finding for the particular case, thus overcoming the “black box” perception of AI.
Introduction
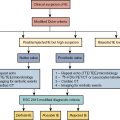
The utilization of computer algorithms to mimic the cognitive functions typically performed by humans is referred to as artificial intelligence (AI). AI-based techniques have emerged as highly efficient tools to help improve the accuracy and efficiency of diagnostic imaging interpretation and, as such, may play a particularly important role in nuclear cardiology. These tools can be integrated to improve image quality, image segmentation, and image quantification. AI has recently revolutionized image analysis. In particular, deep learning (DL), one type of AI, has gained considerable attention recently as a method to accurately classify images. , Although nuclear cardiology analysis has been robustly automated using traditional image processing approaches, AI can further improve automation and accurately extract information directly from cardiovascular images. There is a wealth of imaging and clinical information associated with a typical nuclear cardiology study, which can be challenging to integrate even for experienced clinicians. Machine learning (ML), a subfield of AI, refers to algorithms that assess and learn from observations based on expert-engineered features that are predictive of outcomes to more accurately predict those outcomes in future observations. Novel AI methods, such as DL approaches, extract these features themselves directly from the data. These algorithms are ideally suited to integrate the vast amount of clinical, stress testing, and imaging information from nuclear cardiology studies to improve disease diagnosis or risk prediction. Lastly, explainable AI techniques are being developed to help overcome the traditional perception of AI as a so-called black box by presenting the rationale for the computed decision or recommendation. Novel convolutional neural networks (CNNs), a DL method, can also highlight specific image regions that trigger the AI predictions by creating an attention map. ,
The majority of nuclear cardiology studies are single photon emission computed tomography (SPECT) or positron emission tomography (PET) myocardial perfusion imaging (MPI) for the diagnosis and management of coronary artery disease (CAD). Therefore, these studies will be the primary focus of this chapter. Many SPECT and PET scanners are offered in hybrid configuration with a computed tomography (CT) scanner for attenuation correction; therefore, we will also discuss AI applications in CT. Finally, we will review some practical considerations regarding AI implementation into clinical nuclear cardiology practice.
Using artificial intelligence to improve image processing
Accurate image analysis (e.g., localization, segmentation, quantification) is critical to ensuring the clinical utility of SPECT or PET MPI. Although this process has been automated, AI-based algorithms can improve the precision of this process and reduce the need for time-consuming manual adjustments. Although traditional segmentation algorithms are relatively robust, accurate definition of the mitral valve plane still presents a challenge and frequently requires manual corrections. Betancur et al. proposed a support vector machine (SVM) algorithm for mitral valve plane localization in SPECT MPI, which was validated against the anatomic position of the valve from coronary CT angiography. The fully automated AI-based method had similar Bland-Altman confidence intervals compared with manual adjustment by experienced readers. Wang et al. described preliminary results with an end-to-end fully CNN to segment left ventricular (LV) myocardium by delineating its endocardial and epicardial surfaces. The CNN was trained with LV contours delineated by healthcare providers and demonstrated excellent precision (with an LV myocardium volume error of – 1.1 ± 3.7%).
AI-based techniques have also been developed for image registration of external CT-based attenuation correction maps with SPECT myocardial perfusion data. Ko et al. developed a CNN-based algorithm to achieve automatic coregistration of imaging data. The algorithm was trained to predict the true offset between images in three dimensions (3D) compared with manually coregistered and verified solid-state SPECT MPI and noncontrast CT images. The algorithm was trained in 402 cases and tested in 100 cases (with mean residual misalignment between image pairs 1.71 ± 1.32 mm for the training data set and 2.38 ± 2.00 mm for the testing data set).
Using artificial intelligence to improve image quality
AI could be used to improve the quality of the image reconstruction of nuclear cardiology studies, potentially reducing noise, thereby allowing for shorter imaging times and/or reduction in injected dose to help reduce radiation exposure. DL networks have been demonstrated to automatically detect motion artifacts on coronary calcium scoring in a chest CT. Generative adversarial networks have also been applied to reduce noise in low-dose CT scans, with preliminary work also reported for cardiac PET studies. For example, Ladefoged et al. evaluated the potential of denoising cardiac 18 F-fluorodeoxyglucose (FDG)-gated PET images with a DL approach. The study simulated low-dose imaging by subsampling the full dose imaging data set to only 1% of the injected dose. A total of 146 patients were used to train their DL model, with model testing in a group of 20 patients. Quantitatively, the uncorrected 1% low-dose images showed an average underestimation in end-diastolic and end-systolic volumes of 25%. The bias was nearly removed using the proposed DL denoising method, which led to an average underestimation of only 2%. In simulations, DL could also be used to reduce 18 F–sodium fluoride (NaF) scan times by 10-fold. Ramon et al. demonstrated the feasibility of denoising low-dose SPECT MPI using a 3D convolutional neural network, based on stacked convolutional autoencoders. Images denoised with the CNN, using data simulating one-sixteenth of the clinical dose, achieved similar image quality to simulations using one-eighth of the clinical dose with standard denoising methods. Recently, Song et al. demonstrated a 3D residual CNN trained and tuned in 95 patients, then tested in 24 patients simulating image reconstruction with a fourfold reduction in radiation exposure. Compared with standard dose images, the CNN improved spatial resolution compared with conventional image processing with Gaussian filters or spatiotemporal nonlocal means filtering.
Using artificial intelligence to improve the diagnosis of coronary artery disease
AI-based techniques are also well suited to integrate the large number of clinical variables from nuclear cardiology studies to improve diagnosis. Arsanjani et al. assessed whether the diagnostic accuracy of conventional SPECT MPI could be improved by using an ML approach with SVMs. Changes in myocardial perfusion, assessed with total perfusion deficit (TPD), ischemic changes, and LV ejection fraction, between stress and rest were derived automatically with quantitative software in a test population. Using a parameter grid-search in the training data, SVM models demonstrated improvement in diagnostic accuracy compared with the individual quantitative parameters (86% vs 81%; Fig. 32.1 ). The same group also applied LogitBoost ensemble ML methods to combine clinical and imaging data using 10-fold cross-validation and compared it with the total perfusion deficit and visual scores in a large cohort (N = 1181) with expanded clinical information. LogitBoost achieved significantly higher accuracy (87.3%) for detection of obstructive CAD compared with one of the expert readers (82.1%) or TPD (82.8%), and higher area under the receiver operating characteristic curve (AUC) (0.94) than TPD (0.88) or either expert reader (0.89 and 0.85). Importantly, clinical information significantly improved the AUC achieved by LogitBoost compared with its performance with imaging characteristics alone.

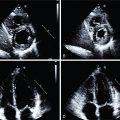
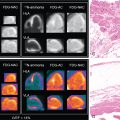
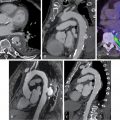
Classical ML has also been applied to predict early revascularization after SPECT MPI, demonstrating results at least as good as expert readers. Indeed, ML outperformed contemporary quantitative methods on a per-vessel and per-patient basis for predicting early revascularization in a large multicenter study. Additionally, the ML algorithm outperformed expert visual interpretation. Figs. 32.2 and 32.3 demonstrate two case examples from this study. In Fig. 32.2 , visual expert interpretation and ischemic TPD identified the study as low risk, but the presence of TID and ST deviation during stress led to a higher ML risk estimation. The patient had an 80% left main stenosis at coronary angiography. In contrast, the example in Fig. 32.3 shows that expert interpretation and quantitative TPD suggested intermediate risk, whereas the ML algorithm suggested low risk. The patient had nonobstructive CAD at coronary angiography. Betancur et al. evaluated the automatic prediction of obstructive CAD from MPI by DL compared with standard quantitative assessment of the total perfusion deficit. With ML thresholds set to match the specificity achieved by TPD, per-vessel sensitivity improved from 64.4% (TPD) to 69.8% (CNN). Although the improvement in specificity was modest, it was obtained using imaging data alone, and further gains would be expected by incorporating clinical and stress testing data. By comparison, attenuation correction leads to around a 3% improvement in diagnostic accuracy. The ML algorithm has the additional benefits that it can be implemented immediately with no change in equipment, protocol, or radiation exposure to patients. Betancur et al. have further developed this technique to consider both upright and supine data, outperforming existing automated quantification methods. The CNN technique was compared with the traditional combined-TPD approach used for analysis of two position data, , in each of the four participating data centers (total of 1160 cases), using a strict external validation regime (leave-one-site-out approach). The CNN-based approach had superior diagnostic accuracy on a per-patient (AUC 0.81 vs. 0.78, p < .001) and per-vessel (AUC 0.77 vs. 0.73, p < .001) basis ( Figs. 32.4 and 32.5 ). Spier et al. implemented methods for abnormality detection with graph CNN using expert visual interpretation as the gold standard. Abnormality localization was performed using 17-segment polar maps, by testing the effect of the missing segment on the final prediction. Deep CNNs have also been applied to PET MPI studies in patients with suspected cardiac sarcoidosis, demonstrating improved sensitivity and specificity compared with quantification with standardized uptake value or coefficient of variation.




Using artificial intelligence to improve risk prediction
Just as ML can combine nuclear cardiology imaging parameters to improve diagnosis, similar methods can be used to improve risk prediction. Betancur et al. applied classical feature-based ML to single-site SPECT MPI data (n = 2619) to determine the benefit of combining clinical and imaging features for major adverse cardiovascular event (MACE) prediction. ML with 10-fold cross-validation had higher AUC for MACE prediction compared with expert visual interpretation, stress TPD, and ischemic TPD (AUC: 0.81 vs. 0.65 vs. 0.73 vs. 0.71, respectively; p < .01 for all) ( Fig. 32.6 ). Importantly, 19% of the studies in the 95th percentile of risk derived by the AI method had been interpreted visually as normal. The overall net risk reclassification for the ML compared with visual interpretation was 26% ( p < .001). These results suggest that AI techniques, incorporating all available clinical and imaging variables, could be an important complementary tool for improving the interpretation of nuclear cardiology studies.