Etiology
Asbestos is the general term given to a group of magnesium silicate minerals that have in common a tendency to separate into fibers. The fibers are resistant to heat and acid, thus the name asbestos, which is derived from the Greek meaning inextinguishable ( a-, “not,” and sbestos, “extinguishable”). Because asbestos is not combustible, has great tensile strength, and is durable, it has been widely used in insulation materials, brake pads and linings, floor tiles, electric wiring, paints, and cements.
Asbestos can be divided mineralogically into two major groups: the serpentines, of which the only member of commercial importance is chrysotile (white asbestos); and the amphiboles, which include amosite (brown asbestos), crocidolite (blue asbestos), and tremolite (a common contaminant of chrysotile). Chrysotile fibers are typically curved, whereas the amphiboles are straight. These physical properties and chemical differences are responsible for the varying uses of asbestos and for their ability to cause disease. Chrysotile is more easily cleared from the lung than the amphiboles, has a weaker fibrinogenic and carcinogenic potential, and is the only type of asbestos fiber that is still widely used. The current products are mainly cements, friction materials, and plastics.
Prevalence and Epidemiology
The widespread use of asbestos in the first 7 decades of the 20th century resulted in an epidemic of asbestos-related illness that continues into the 21st century in spite of a major decline in use after documentation of its major hazards, particularly for amphibole asbestos fibers (crocidolite and amosite) in the 1970s and 1980s. Although the use of asbestos has now been banned in many Western countries, the prevalence of asbestos-related pulmonary and pleural complications has been increasing. For example, surveys in the United States and in Europe have shown a doubling of the prevalence of asbestos-related pleural complications, including mesothelioma, between the early 1970s and 2000. Similarly, whereas mortality rates for the other pneumoconioses decreased between 1968 and 2000, the mortality of asbestosis increased steadily, and it is now the most frequently recorded pneumoconiosis on death certificates. It has been estimated that 8 to 9 million people in the United States have had occupational exposure to asbestos and that such exposure will eventually result in 300,000 deaths. These complications are still occurring because the interval between initial exposure and subsequent biological consequences of asbestos exposure is variable, ranging from approximately 1 year for some cases of pleural effusion to more than 40 years for mesothelioma.
The four main settings in which asbestos-related disease is seen in the Western world are
- 1.
The historical legacy of asbestos exposure affecting older workers
- 2.
The current risk experienced by the work force engaged in certain occupations managing the remaining asbestos hazard, such as building and facility maintenance
- 3.
Asbestos abatement operations for removal of insulation and other asbestos-containing products
- 4.
Renovation and demolition of structures containing asbestos
Most asbestos in North America today exists in building and machinery insulation and old products, such as appliances. New products that may contain asbestos today in the United States include friction surfaces (brake pads), roofing materials, vinyl tiles, and imported cement pipe and sheeting. It is estimated that asbestos is currently still a hazard for approximately 1.3 million workers in the construction industry in the United States and for workers involved in maintenance of buildings and equipment. The risk of asbestos exposure is considerably higher in countries where it continues to be used. Currently, the majority of asbestos is mined in Russia, with other mining operations existing in China, Brazil, and Kazakhstan. Until 2011 Quebec, Canada, operated two asbestos mines, but those mines are now closed despite a 2012 legislative attempt to restart one of the mines.
Clinical Presentation
Most patients who have asbestos-related pleuropulmonary disease are asymptomatic. Patients with asbestosis, chronic airflow obstruction, or diffuse pleural fibrosis may present with shortness of breath. The onset of dyspnea in asbestosis is typically insidious, beginning with dyspnea on exertion. The dyspnea related to asbestosis is generally progressive, even in the absence of continued dust exposure. In patients who have asbestosis, shortness of breath seldom occurs sooner than 20 to 30 years after initial exposure. A nonproductive cough is also commonly present. Pleuritic chest pain may accompany the development of benign asbestos effusion or mesothelioma. Benign asbestos effusions are generally less than 500 mL in volume, are often serosanguineous, and may persist for 2 weeks to 6 months. They are recurrent in 15% to 30% of cases. The presence of pleural effusion or diffuse pleural thickening should raise the possibility of mesothelioma.
Pathophysiology
The pathogenesis of asbestos-related pleuropulmonary disease is complex and incompletely understood. Fiber dose, dimension, and chemical composition may all influence fibrogenicity and carcinogenicity; longer, thinner, more durable fibers (amphiboles) are the most important. Factors related to the host, including pulmonary clearance, immunologic status, and exposure to other noxious substances, such as cigarette smoke, are also important in determining the nature and severity of the reaction to inhaled fibers.
Asbestos fibers are deposited at airway bifurcations and in respiratory bronchioles and alveoli. They migrate into the interstitium, in part through an uptake process involving type I alveolar epithelial cells. Once they reach the interstitium, the asbestos fibers cause an alveolar macrophage-dominated alveolitis, inflammation in the surrounding interstitium, and inflammation followed by fibrotic change in the respiratory bronchioles that extends into the adjacent alveolar tissue.
Pathologic Characteristics
Asbestos-Related Pulmonary Abnormalities
Asbestosis.
The earliest pulmonary abnormality seen in association with asbestos exposure consists of fibrosis in the walls of respiratory bronchioles ( Fig. 61.1 ). This process, however, is pathogenetically distinct from pulmonary parenchymal fibrosis and may be seen in patients with a history of exposure to mineral dust other than asbestos. Peribronchiolar fibrosis is probably better considered a form of asbestos airway disease separate from asbestosis. Asbestosis itself is defined as diffuse interstitial pulmonary fibrosis caused by the inhalation of excessive amounts of asbestos fibers. The interstitial fibrosis involves mainly the lower zones; the worst disease is generally seen closest to the pleura, with relative sparing of the central portions of the lung. The microscopic appearance varies from a slight increase in interstitial collagen to complete obliteration of normal lung architecture and the formation of thick fibrous bands and honeycombing. The microscopic diagnosis of asbestosis is based on the presence of two findings: diffuse interstitial fibrosis, which in advanced cases is identical to that seen in usual interstitial pneumonia, and asbestos bodies, fibers of asbestos to which the lung has added an iron-protein coating ( Figs. 61.2 and 61.3 ). The development of asbestosis requires heavy exposure to asbestos and therefore is seen mainly in asbestos miners and millers, asbestos textile workers, and asbestos insulators. Fibrosis of adjacent visceral pleura is common and often accompanied by parietal pleural adhesions. Asbestosis is commonly associated with prolonged exposure, usually over 10 to 20 years, and the degree of fibrosis is dose dependent. Transport of the asbestos fibers to the pleural surface along lymphatic channels or by direct penetration results in pleural inflammation and fibrosis.



Rounded Atelectasis.
Rounded atelectasis, also known as folded lung, consists of a more or less spherical focus of collapsed parenchyma in the periphery of the lung. The overlying pleura is invariably fibrotic and shows one or more invaginations 1 mm to 3 cm in length into the adjacent lung. Although rounded atelectasis is not related solely to asbestos, the majority of cases are associated with it. The lesion is believed to develop secondary to infolding of thickened visceral pleura with atelectasis of the intervening lung parenchyma. Rounded atelectasis may be multiple and bilateral.
Lung Cancer.
Asbestos exposure is associated with an increased risk of lung cancer, and this risk is markedly increased in smokers (supraadditive effect—an effect greater than the sum of either risk alone). Amphiboles, particularly crocidolite, are more potent than chrysotile in inducing lung cancer (between 10 and 50 times greater potency). The risk of lung cancer in commercial chrysotile is believed to be largely determined by the varying content of tremolite. The mechanism of carcinogenesis is unclear. The latent period is variable. Some cases occur less than 10 years after exposure, but the majority of cases occur much later. In a study of 3383 asbestos-exposed workers, 63 (1.9%) had pulmonary carcinoma; the mean latency period for the development of carcinoma was 45.8 ± 9.4 years, and the mean age of patients was 67.6 ± 8.4 years. Asbestos-related cancers can occur anywhere in the lungs and be of any cell type.
Although it is clear that asbestos exposure is associated with an increased incidence of lung cancer, it is controversial whether asbestos-related lung cancer arises only in the presence of pulmonary fibrosis. The various epidemiologic studies assessing the carcinogenic role of asbestos in the presence and absence of fibrosis were recently reviewed, and the conclusion of the reviewers was that the evidence is not conclusive and that the “question of whether or not asbestos-related lung cancer in man arises only in the presence of pulmonary fibrosis may be unanswerable epidemiologically.”
Asbestos-Related Pleural Abnormalities
Pleural Plaques.
These are the most common form of asbestos-related pleuropulmonary disease. They are usually first seen 20 to 30 years after exposure. Pleural plaques consist of well-defined, pearly white foci of firm fibrous tissue, usually 2 to 5 mm thick and up to 10 cm in diameter. The major determinant of the thickness of the pleural plaques is duration from first exposure. The plaques may have a smooth surface or show fine or coarse nodularity and can be round, elliptical, or irregularly shaped. Histologic examination typically shows them to consist of dense, almost acellular collagen with a basket-weave pattern ( Fig. 61.4 ). Pleural plaques are usually limited to the parietal pleura, although they may occasionally be seen in the interlobar fissures. Approximately 10% to 15% of pleural plaques calcify. Calcification is seldom evident in workers with a less than 30-year interval from time of first exposure. The calcification may be punctate, linear, or coalescent.

Pleural Effusion.
Although only a small percentage of individuals exposed to asbestos develop a benign pleural effusion (3%), this is the most common asbestos-related abnormality seen within the first 20 years after exposure. The effusion is exudative and often hemorrhagic. It may occur as early as 1 year after exposure or much later. Although usually asymptomatic, acute pleural effusions caused by asbestos may also be exuberant, with fever and severe pleuritic pain. Acute pleuritis is thought to underlie many cases of diffuse pleural thickening, with studies demonstrating diffuse pleural thickening in patients with prior benign asbestos effusion in 31% to 80% of patients.
Diffuse Pleural Thickening.
Diffuse pleural thickening extends continuously over a portion of the visceral pleura and therefore is quite different in appearance from the circumscribed parietal pleural plaque. It may be extensive and cover the whole lung and obliterate lobar fissures; it ranges from less than 1 mm up to 1 cm or more in thickness. Adhesions to the parietal pleura are common. The thickening may extend for a few millimeters into the lung parenchyma and into the lobular septa. These features do not constitute asbestosis. Diffuse pleural thickening is seen in 9% to 22% of asbestos-exposed workers with pleural disease. The frequency of diffuse pleural thickening increases with time from first exposure and is believed to be dose related. Diffuse pleural thickening frequently develops after benign asbestos-related pleural effusion. Less commonly, it may be caused by extension of interstitial fibrosis to the visceral pleura, consistent with the pleural migration of asbestos fibers. Both circumscribed (pleural plaques) and diffuse pleural thickening may be present in the same hemithorax.
Mesothelioma.
There is a strong association between asbestos exposure and the development of malignant mesothelioma; the majority of cases of mesothelioma are related to asbestos exposure. The risk of mesothelioma is much greater after exposure to amphiboles, particularly crocidolite (blue asbestos), than after exposure to chrysotile (white asbestos). Although it was initially believed that the risk of mesothelioma from exposure to chrysotile was due to contamination with the amphibole tremolite, similar to other lung cancers, current evidence suggests that chrysotile itself may cause malignant mesothelioma, although the risk is much lower than for amphiboles. Mesothelioma has occurred in three main cohorts of asbestos-exposed individuals: workers exposed to asbestos during its mining or milling; workers exposed in the manufacture and use of asbestos products, such as plumbers, carpenters, and installers of asbestos insulation; and persons who were exposed to asbestos unknowingly and incidentally. The last group accounts for 20% to 30% of current cases of malignant mesothelioma in industrialized countries.
The incidence of mesothelioma has markedly increased during the past 30 years, currently being as common in men as carcinomas of the liver, bone, and bladder in many industrialized countries. In countries no longer using asbestos, the incidence of mesothelioma is now expected to start decreasing. However, in developing countries that have recently used asbestos or continue to use it, the rate of mesothelioma may continue to increase. The risk of mesothelioma is almost zero for the first 10 to 15 years after first exposure to asbestos but increases progressively thereafter throughout life. Although the role of asbestos in the development of mesothelioma has been extensively studied, and several explanations have been proposed as to how asbestos may result in the development of malignant disease, the pathogenesis of mesothelioma remains unclear. See Chapter 74 for additional discussion of mesothelioma.
Pulmonary Function Tests
Patients with asbestosis may have restrictive lung function, mixed restrictive and obstructive disease, or airflow obstruction alone. When restriction is present, total lung capacity, vital capacity, residual volume, and diffusing capacity are decreased, but good ventilatory function is maintained. Many patients, however, show some degree of airway obstruction as a result of asbestos-induced bronchiolar fibrosis. In addition, emphysema is evident on computed tomography (CT) scans in about 50% of workers who have early asbestosis, a figure double that of those who do not.
Restrictive lung function in asbestos workers may result from asbestosis or from asbestos-related pleural disease. The lung restriction (i.e., the decrease in vital capacity) is much more marked in patients who have diffuse pleural thickening than in those who have only plaques. This effect is unrelated to the radiographic extent of pleural thickening; a reduction in vital capacity with little more than costophrenic angle blunting can be similar to that with extensive involvement.
Manifestations of the Disease
Radiography
Interpretation According to the International Classification of Radiographs of the Pneumoconioses
The chest radiograph is an important tool for the detection of asbestos-related pleural and parenchymal abnormalities and to assess the progression of ensuing disease. For it to be useful in epidemiologic studies, however, it is essential that an acceptable classification of the extent of involvement be followed and a standard nomenclature be used. The most widely used schema is the International Labour Office (ILO) International Classification of Radiographs of Pneumoconioses, which was revised most recently in 2011. The objective of this classification is to codify the radiographic changes associated with the pneumoconioses in a simple and reproducible manner such that international comparability of pneumoconiosis statistics has some validity. Because this schema uses radiographic descriptors that are somewhat different from those generally used throughout this book, a short glossary of terms relevant to the interpretation of radiographs of asbestos-exposed workers and other pneumoconiosis patients follows.
Small Rounded Opacities.
These opacities are well-circumscribed nodules ranging in diameter from barely visible to up to 10 mm. The qualifiers p, q, and r subdivide the predominant opacities into three diameter ranges: p, up to 1.5 mm; q, 1.5 to 3.0 mm; and r, 3 to 10 mm.
Small Irregular Opacities.
This term is used to describe a pattern that elsewhere in this book has been designated linear or reticular —in other words, a net-like pattern. Although the nature of these opacities is such that establishment of quantitative dimensions is considerably more difficult than with rounded opacities, three categories have been created: s, width up to 1.5 mm; t, width exceeding 1.5 mm and up to 3.0 mm; and u, width exceeding 3 mm and up to 10 mm.
To record shape and size, two letters must be used. If the reader of the radiograph thinks that all or virtually all opacities are one shape and size, this is noted by recording the symbol twice, separated by an oblique stroke (e.g., q / q ). If another shape or size is appreciated, this is recorded as the second letter (e.g., q / t ). The designation q / t means that the predominant small opacity is round and of size q, but in addition there are a significant number of small irregular opacities of size t. In this way, any combination of small opacities can be recorded.
Profusion.
This term refers to the number of small opacities per unit area or zone of lung. There are four basic categories: category 0, small opacities absent or less profuse than in category 1; category 1, small opacities definitely present but few in number (normal lung markings are usually visible); category 2, numerous small opacities (normal lung markings are generally partly obscured); and category 3, very numerous small opacities (normal lung markings are usually totally obscured). These categories can be further subdivided by use of a 12-point scale to describe a continuum of changes from complete normality to the most advanced category or grade: 0/− 0/0 0/1; 1/0 1/1 1/2; 2/1 2/2 2/3; 3/2 3/3 3/+. When this scale is used, the radiograph is first classified into one of the four categories 0, 1, 2, or 3. If the category above or below is considered a serious alternative during the process, it is recorded (e.g., a radiograph in which profusion is considered to be category 2 but for which category 1 was seriously considered would be graded 2/1). If no alternative is considered (i.e., the profusion was definitely category 2), it would be classified 2/2. A subdivision is also possible within category 0. If the absence of small opacities is particularly obvious, profusion should be recorded as 0/−. Such a category might be seen in a healthy nonsmoking adolescent. The ILO standard radiographs are the final arbiters of opacity profusion and take precedence over any application of a verbal description of profusion. Thus this reading should always be done side by side with the ILO standard radiographs.
A separate classification for large opacities (>1 cm in diameter) exists whereby A denotes one or more opacities greater than 1 cm but smaller than 50 mm; B denotes one or more opacities greater than A, combined area less than the right upper zone; and C denotes one or more opacities whose combined area is greater than the area of the right upper zone.
Other Findings.
Pleural abnormalities are also assessed with respect to location, width, extent, and degree of calcification. Finally, other abnormal features of the chest radiograph can be noted. All of these abnormalities are more completely illustrated and described in the Guidelines for the Use of the ILO International Classification of Radiographs of Pneumoconioses.
There is little doubt that expert readings are superior to nonexpert readings in the detection of disease and avoidance of “overreading.” However, use of the radiograph as a definitive tool for the diagnosis of pneumoconiosis in any given individual is limited by a significant prevalence of small opacities sufficient for “diagnosis” in nonexposed working populations, as well as by a significant degree of interreader and intrareader variability.
Asbestos-Related Pulmonary Abnormalities
Asbestosis.
The initial radiographic manifestation of asbestosis consists of bilateral small irregular linear opacities (reticular pattern, s and t opacities in the ILO classification) in the lower lung zones. With progression of disease, the distribution and extent or profusion of opacities increases and may spread to the middle and upper lung zones ( Fig. 61.5 ). Fibrosis in the right middle lobe and lingula may result in obscuration of the heart border (“shaggy heart sign”). Extensive fibrosis was more common in the past, with more patients currently having only mild fibrosis. In a study of 3383 asbestos-exposed workers referred for independent medical evaluation, most subjects (79%) had an ILO score below 1/1.

The extent of parenchymal abnormalities (ILO classification profusion score) correlates strongly with functional impairment, particularly reduction in diffusing capacity and ventilatory capacity, and with mortality risk. In the assessment of radiographs of asbestos-exposed individuals, a critical distinction is made between radiographs that are suggestive but not presumptively diagnostic (0/1) and those that are presumptively diagnostic but not unequivocal (1/0). This dividing point is generally taken to separate radiographs that are considered to be “positive” for asbestosis (1/0 or higher profusion score) from those that are considered to be “negative” (0/1 or less).
A chest radiograph showing the characteristic findings of asbestosis in the presence of a compatible history of exposure is adequate for the diagnosis; further imaging is not required. However, the chest radiograph has limited sensitivity and specificity in cases of mild fibrosis and may not provide sufficient additional information, such as degree of progression from prior imaging, for use in a given clinical case.
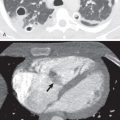
Rounded Atelectasis.
The characteristic radiologic manifestation of rounded atelectasis is a rounded or oval, subpleural opacity associated with loss of volume and curving of adjacent pulmonary vessels and bronchi into the edges of the opacity (the “comet tail sign”; Fig. 61.6 ). The opacity typically abuts an area of pleural thickening or a pleural effusion. The abnormality may occur anywhere in the lungs, but it is most common in the middle and lower lung zones (see Fig. 61.6 ). In one review of 89 cases of rounded atelectasis in 74 patients, the lower lobes were affected in 33 cases (45%), the lingula in 33 (45%), the middle lobe in 16 (22%), and the upper lobes in 7 (9%). Rounded atelectasis may be unilateral or bilateral and usually measures 2 to 7 cm in diameter.

Most cases of rounded atelectasis are associated with asbestos exposure; however, some have been described in association with other causes of pleural thickening or effusion. The lesion may develop and progress during a few months or several years. In a series of 74 patients, it occurred on a background of benign asbestos effusion in 9 and slowly increasing pleural thickening in 13; in the remaining 52 patients, it was a new finding, with earlier radiographs showing only plaques or being normal.
Asbestos-Related Pleural Abnormalities
Pleural Plaques.
Pleural plaques are the most common radiologic manifestation of asbestos-related disease. The characteristic findings on the chest radiograph consist of focal pleural opacities that have irregular margins when seen in profile and sharp, often foliate borders when seen en face ( Fig. 61.7 ). The typical distribution of plaques on chest radiographs is the posterolateral chest wall between the 7th and 10th ribs, the lateral chest wall between the 6th and 9th ribs, the dome of the diaphragm (virtually diagnostic), and the paravertebral pleura ( Fig. 61.8 ). The plaques may be bilateral and symmetric or bilateral and asymmetric and less commonly appear unilateral on the chest radiograph. They may be smooth or nodular and can measure as much as 1 cm in thickness. Although noncalcified pleural plaques are the most common radiographic manifestation of asbestos-related disease, they are more striking when calcified, a finding that is seen in 10% to 15% of cases ( Figs. 61.9 and 61.10 ). Although calcified plaques may be seen at any location, they are most common in relation to the diaphragm. The prevalence of pleural plaques is related to duration from first exposure; they are rare before 20 years. Calcification of pleural plaques is similarly related to duration and is more common 30 years after first exposure (see Fig. 61.10 ).





Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree