CONGENITAL HEART
DEFECTS
2
 INTRODUCTION
INTRODUCTION
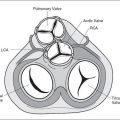
The fetal heart undergoes complex developmental changes in the first few weeks of gestation and is anatomically fully developed by the eighth week of embryonic life (1). The presence of congenital heart defects in the fetus is a result of abnormal cardiac development during its embryogenesis. Current knowledge suggests that the genetic contribution to congenital heart defects has been significantly underestimated in the past. Recent advances in microscopic techniques have added significantly to the knowledge of early cardiac development and how congenital heart defects originate (2). Human cardiovascular genetics is a field that is progressing at a rapid pace, and clinically available genetic tests for various forms of cardiac abnormalities are made available at variable speed (3). Online resources listed at the end of this chapter will provide an updated list of available genetic testing. Table 2-1 displays, in chronologic order, important milestones in fetal cardiac development, and Table 2-2 lists associated cardiac defects with the corresponding stage of cardiac development.
 CONGENITAL HEART DEFECTS AND NUMERICAL CHROMOSOMAL ABNORMALITIES
CONGENITAL HEART DEFECTS AND NUMERICAL CHROMOSOMAL ABNORMALITIES
The frequency of chromosomal abnormalities in infants with congenital heart defects has been estimated as 5% to 15% from postnatal data (4–6). In a population-based case-control study of 2102 live-born infants, ascertained by their cardiovascular malformations, chromosomal abnormalities were found in 13% (5). In this study, Down syndrome occurred in 10.4% of infants with cardiovascular malformations, with the other trisomies each occurring in less than 1% of cases (5). Similar data were reported from three large registries of congenital malformations, involving 1.27 million births (6). The frequency of abnormal karyotype in fetuses with cardiac defects is higher and has been reported in the range of 30% to 40% by several studies (7–9). This higher rate of chromosomal abnormalities in fetuses with cardiac defects, when compared to their live-born counterparts, is mainly due to an increased prenatal mortality in fetuses with aneuploidy, which has been estimated at 30% for trisomy 21, 42% for trisomy 13, 68% for trisomy 18, and 75% for Turner syndrome (10). Not only is the association of congenital cardiac defects and chromosomal abnormalities lower in live-born infants when compared to fetuses, but also the distribution of chromosomal abnormalities is more skewed toward Down syndrome in the neonatal population (5,6), again probably due to the high prenatal mortality of trisomy 18, trisomy 13, and monosomy X.
Certain specific cardiac diagnoses are more commonly associated with chromosomal abnormalities than others. Prenatal and postnatal studies are concordant with regard to the specific cardiac diagnoses that are more likely to be associated with chromosomal abnormalities. In general, malformations of the right side of the heart are less commonly associated with karyotypic abnormalities. Specific cardiac diagnoses like transposition of the great vessels and heterotaxy syndromes are not usually associated with chromosomal abnormalities. Endocardial cushion defect, ventricular (perimembranous) and atrial septal defects, tetralogy of Fallot, double outlet right ventricle, and hypoplastic left heart syndrome, on the other hand, are more commonly associated with chromosomal abnormalities in the fetus and newborn. Table 2-3 lists specific cardiac diagnoses in infants with noncomplex cardiovascular defects from three large registries (6) and the corresponding incidence of associated numerical chromosomal abnormalities.
The majority of fetuses with cardiac defects and chromosomal abnormalities have other associated extracardiac abnormalities, in the order of 50% to 70% (7,9). The distribution of extracardiac abnormalities usually follows the typical pattern noted within each chromosomal syndrome with no predominance of any specific abnormality. In the fetus with an apparently
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree