Key Points
- ▪
Although echocardiography and MRI are still the best overall modalities for the evaluation of aortic and complex congenital heart lesions, especially postoperatively, there are exceptions.
- ▪
A growing number of children and adults with congenital disease acquire contraindications to cardiac MRI, such as pacemakers and implantable cardioverter defibrillators.
- ▪
Many surgical and interventional procedures use metallic components such as stents, occluder devices, and bioprosthetic valves, which often can confound MR imaging. In these cases CT may be beneficial.
- ▪
Although retrospective imaging is higher in radiation dose, it allows accurate measurement of right and left ventricular size and function.
- ▪
Using technique optimization, a number of complex congenital heart lesions can be well imaged with CT angiography.
- ▪
Surgical corrections for congenital heart disease can be well assessed with CT angiography. Upper and lower extremity contrast opacification may be necessary, as well as delayed imaging, especially if intracardiac thrombi are to be excluded.
The established tests for the assessment of congenital heart disease are transthoracic and transesophageal echocardiography, cardiac MRI, and cardiac catheterization.
Cardiac CT (CCT) is an emerging alternative, because of its rapid acquisition times and post-processing robustness. The potential radiation risks are particularly relevant for children and younger adults, especially because its use has increased prominently in the assessment of congenital heart disease.
Most intracardiac lesions can be assessed by echocardiography, and many procedures can be planned and guided with transthoracic, transesophageal, or intracardiac echocardiography, avoiding radiation risk and also providing Doppler assessment.
Among the patient population with congenital heart disease, MRI is the established test for the evaluation of extracardiac surgical shunts, pulmonary vascular anomalies and complications, and aortic anomalies. This modality avoids the risk of radiation exposure, and also provides flow information. Cardiac MRI also is the preferred test to quantify right ventricular (RV) function and the degree of pulmonic insufficiency.
CCT provides nonphysiologic but superb anatomic delineation of a very wide range of congenital cardiac and thoracic vascular defects, and its use has substantially increased, but it remains unclear which lesions should be assessed by CCT, given the established, radiation risk–free, and widespread availability of other modalities, and the proven complementarity of echocardiography and cardiac MRI.
The greatest contribution of CCT, therefore, is likely to be:
- □
In patients who cannot undergo MRI, or those with lesions such as extracardiac shunts in whom MRI images are insufficient
- □
For the assessment of coronary anatomy, such as identification of anomalous left anterior descending (LAD) coronary artery in patients with tetralogy of Fallot
A number of congenital pathologies are suited for CT imaging (see Table 25-1 ). The most common are discussed in the following sections. It may be useful to categorize congenital lesions based on their simplicity rather than on physiology.
| APPROPRIATENESS RATING | INDICATION | MEDIAN SCORE |
|---|---|---|
| Appropriate | Assessment of anomalies of coronary arterial and other thoracic arteriovenous vessels | 9 |
| Assessment of complex adult congenital heart disease | 8 | |
| Quantitative evaluation of right ventricular function | 7 | |
| Uncertain | None listed | |
| Inappropriate | None listed |
Complex Lesions
Lesions that may be described as complex include the following:
- □
Tetralogy of Fallot
- □
Postsurgical tetralogy of Fallot
- □
Transposition of the great arteries
- □
Postsurgical transposition of the great arteries
- •
Mustard baffles
- •
Jatene’s arterial switch operation
- •
- □
Congenitally corrected transposition of the great arteries
- □
Fontan procedure
- •
Glenn shunt
- •
- □
Other commonly performed surgical repairs
- •
Blalock-Taussig shunt
- •
Waterston-Cooley shunt
- •
Pott’s shunt
- •
Rastelli procedure
- •
Tetralogy of Fallot
Tetralogy of Fallot is a common type of complex congenital heart disease. With an incidence of 0.1 per 1000 live births, it occurs in approximately 5.5% of all patients with congenital heart disease. The underlying abnormality is anterior displacement of the infundibular septum, which results in the three basic malformations that characterize this disorder:
- □
Severe stenosis of the right ventricular outflow tract (RVOT)
- □
An overriding aorta
- □
Infundibular venticular septal defect (VSD)
The fourth element of the tetralogy is hypertrophy of the right ventricle, which occurs as a consequence of the three basic defects just cited.
Over the years, the surgical approach to this condition has changed. There has been a move from a staged approach in favor of a primary repair, with a progressive lowering of the age at repair and a surgical technique that avoids or reduces the need for a ventriculotomy.
The goals of surgical correction of tetralogy of Fallot are to relieve the RVOT obstruction and to close the underlying VSD.
The RVOT obstruction can be relieved in one of three ways:
- □
Resection of a portion of the infundibular septum and other structures contributing to the outflow tract obstruction, such as prominent muscle bundles or muscular trabeculae
- □
Enlarging the pulmonary outflow tract by placement of a transannular patch, which is constructed from prosthetic material or from autologous pericardium. This patch is applied to the anterior aspect of the RVOT.
- □
Placement of an external conduit, usually involving a prosthetic pulmonary valve, between the right ventricle and the pulmonary trunk. This often is performed with a Dacron or Gore-Tex conduit and a bioprosthetic valve.
With all three methods, the VSD is closed with a patch of prosthetic material in such a way that the overriding aorta valve is exclusively committed to the left ventricular outflow tract.
Unfortunately, despite the improved surgical management, complications and residual sequelae after repair of tetralogy of Fallot are still common. Residual or recurrent VSD, RVOT obstruction or aneurysm formation, and pulmonary artery regurgitation and/or stenosis all lead to significant right ventricular dysfunction, resulting in significant morbidity and premature mortality.
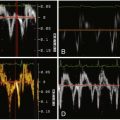
MRI is currently the gold standard tool for postoperative evaluation of repaired tetralogy of Fallot. A number of patients, however, have relative contraindications to cardiac MRI, including pacemakers, claustrophobia, and underlying vascular stents, which can be difficult to image well due to susceptibility artifact.
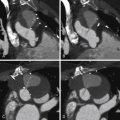
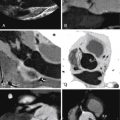
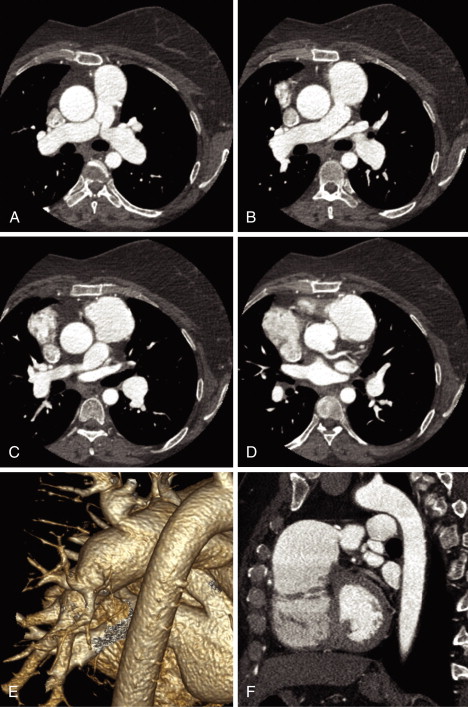
Gated cardiac CT of postoperative tetralogy of Fallot patients allows:
- □
Demonstration of residual anatomic problems:
- •
Residual VSD if present
- •
Pulmonary arterial stenosis (with or without stenting), which can be central or peripheral
- •
RVOT aneurysm
- •
- □
Monitoring
- •
Any underlying ascending aortopathy
- •
Aortopulmonary collaterals
- •
- □
Quantification of right/left ventricular size and function
See Figures 25-1 through 25-3 ;


Postsurgical Tetralogy of Fallot
Most patients with tetralogy of Fallot will have had surgery. Corrective surgery for tetralogy of Fallot can include the following procedures:
- □
Placement of a ventricular septal patch, closing the high ventricular septal defect
- □
Resection of RVOT/infundibular muscle bundles, which usually are the cause of RVOT obstruction
- □
Pulmonic valvotomy. Pulmonic valve tissue often is dysplastic, thickened, and dysfunctional.
- □
Placement of an RVOT patch, often in conjunction with RVOT muscle bundle resection to increase the volume of the RVOT
- □
Transannular repair with placement of a transannular patch. This procedure is performed when the pulmonary valve annulus is small and restrictive. The surgery leaves the patient with free pulmonary insufficiency.
- □
Pulmonic valve implantation. A porcine bioprosthesis or human homograft usually is chosen. These can be utilized in adults undergoing late repair, and in patients with prior pulmonary valvotomy/transannular patch placement who have developed severe RV dilatation.
In patients who have severe hypoplasia, or atresia of the RVOT, an extracardiac conduit can be placed. This usually extends from the RVOT or the body of the RV to a central pulmonary artery.
Most imaging follow-up for tetralogy of Fallot repair is done with echocardiography and cardiac MRI. In patients with contraindications to cardiac MRI, such as a pacemaker, or the patient’s inability to undergo an MRI study due to body habitus or claustrophobia, a CCT study can be of benefit.
While evaluation of pulmonic insufficiency and tricuspid insufficiency is, at best, limited with CT imaging, accurate morphologic evaluation of the right and left cardiac chambers, RV and LV volumes and ejection fractions, and central and peripheral pulmonary arterial anatomy can be obtained with high accuracy using a low-dose, dose-modulated helical acquisition. To assess the right ventricle as well as the left ventricle adequately on a CCT study, increased density of contrast is needed on the right side of the heart. This makes evaluation for intracardiac shunts such as an underlying patent foramen ovale or a VSD patch leak more challenging.
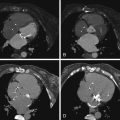
A CCT study for postoperative evaluation of a patient with tetralogy of Fallot repair would include the following:
- □
Determination of RV end-diastolic volume (EDV), RV end-systolic volume (ESV), RV ejection fraction (RVEF)
- □
Left ventricular EDV, left ventricular ESV, left ventricular EF
- □
Evaluation of the intraventricular septum for patch integrity
- □
Evaluation of the RVOT
- □
Evaluation of the native or bioprosthetic pulmonary valve
- □
Evaluation of an RV-to-PA conduit if one is present
- □
Evaluation of central right and left pulmonary arteries
- □
Evaluation of peripheral pulmonary arteries
- □
Evaluation of the ascending aorta, which can become dilated in the setting of tetralogy of Fallot
- □
See Figures 25-4 through 25-6 ;