Key Points
- ▪
Contrast-enhanced CT scanning has developed to afford excellent noninvasive angiography, and also affords imaging of organs and other structures, unlike conventional angiography.
- ▪
Atherosclerotic calcium can be a problem.
- ▪
Knowledge of the range of normal and collateral anatomy is critical.
Noninvasive assessment of carotid artery disease is desirable, as more than half of the permanent morbidity and mortality of the Asymptomatic Carotid Artery Surgery (ACAS) Trial was attributable to catheter-based angiography (1.3% of the 2.3% with permanent morbidity and mortality).
The utility of multidirectional CT (MDCT) for the assessment of cerebrovascular, renal artery, and peripheral arterial disease is less studied and published than is its use for the assessment of coronary disease.
Cerebrovascular and peripheral arterial disease are much less subject to motion, and therefore less taxing of the limited temporal resolution of MDCT, than is coronary artery disease. However, both carotid artery disease and peripheral arterial disease are subject to at least as much calcification as is coronary artery disease. In addition, adjacent bony artifacts (to the popliteal artery from the femur and tibial head) are substantial problems for the MDCT assessment of peripheral arterial disease, and tracking a contrast bolus without getting ahead of it is a protocol challenge for the assessment of peripheral arterial disease.
The renal arteries are subject to motion due to excursion of the adjacent diaphragm, occasionally contributing motion artifacts on MDCT imaging.
Renal Artery Disease
Assessment of the functional significance of renal artery disease is not simple, by any modality, given the complex anatomy of renal arteries, their known anatomic variants, the variability of lesion numbers and locations, and the fact that “renal artery disease” is more of a medical (over)-simplification than a single pathologic entity.
The renal arteries arise 1 cm below the origin of the superior mesenteric artery (SMA). The right renal artery is longer than the left renal artery, as the aorta lies to the left side of the spine. The main renal arteries divide into five segmental renal arteries—superior/apical, posterior, anterior superior/upper, anterior inferior/middle, and inferior/lower—which supply the kidney.
Furthermore, numerous variants or anomalies of renal arterial supply occur:
- □
Multiple accessory or supernumerary renal arteries
- □
“Polar” or “extra-hilar” renal arteries
- □
Accessory renal artery arising at the superior aspect of a kidney
- □
Accessory renal artery arising at the inferior aspect of a kidney
- □
Common adrenal/renal artery
- □
Common inferior phrenic/renal artery
- □
Renal branch from an adrenal artery
- □
Passing anterior to the inferior vena cava (IVC) (right-sided)
- □
Passing posterior to the IVC (right-sided)
- □
Proximal ramification/subdivision of a renal artery
When compared with digital subtraction angiography (DSA), neither CT angiography (CTA) nor MR angiography (MRA) appears able to reliably exclude renal artery stenosis ( Table 27-1 ).
| CTA | MRA | |
|---|---|---|
| Interobserver agreement | 0.59–0.64 | 0.40–0.51 |
| Sensitivity | 64% [55–73%] | 62% [54–71%] |
| Specificity | 92% [90–95%] | 84% [81–87%] |
Given the concurrent existence of renal insufficiency in renal artery disease, the nephrotoxicity of CT contrast material is a real concern for the routine use of CTA for the assessment of renal artery disease—a concern that is shared with contrast angiography, but not with MRA or ultrasound.
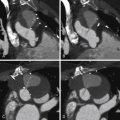
Approximately one third of the general population demonstrates variability in number, location, and branching patterns of the renal arteries. This is clinically important because renal artery stenosis (RAS) in an accessory renal artery can, even though rarely, be responsible for renovascular hypertension. Lesions causing stenosis of more than 50% of the diameter of the artery are considered significant. Although there are no clear-cut indications for intervention, the following criteria may be used as a guide for renal artery revascularization: recent onset of hypertension, in which the goal is to cure the hypertension; drug-refractory hypertension; intolerance to antihypertensive medications; progressive renal insufficiency/failure; and episodes of flash pulmonary edema.
In addition to atherosclerotic renal artery stenosis, another notable cause of renovascular hypertension is fibromuscualr dysplasia (FMD). FMD is a nonatherosclerotic angiopathy of unknown etiology, although a genetic association with possible autosomal dominant transmission has been suggested.
FMD can involve the intima, media, and adventitia, with medial FMD representing the most common type, and is characterized by the classic “string of beads” appearance. FMD usually affects females between 15 and 50 years of age, frequently involves the mid or distal segments of the renal artery, and is bilateral in two thirds of patients. It is the most common cause of renovascular hypertension in children. Renal artery stenosis secondary to FMD may affect pregnant women and thus remains an important consideration as a cause of secondary hypertension during pregnancy.
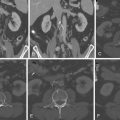
For images of renovascular disease, see Figures 27-1 to 27-3 .



CT Protocol Points
- □
Coverage: arterial phase, above diaphragm to symphysis pubis
- □
Contrast: 80 mL at 5 mL/sec
- □
Automated trigger set:
- •
location: upper abdominal aorta
- •
HU: 180
- •
Peripheral (Lower Extremity) Arterial Disease
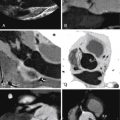
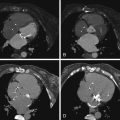
Assessment of peripheral (lower extremity) artery disease by MDCT is feasible but it is challenged by:
- □
The huge anatomic territory to assess with thin (submillimeter) slices; hence the enormous number of images to review (∼1500)
- □
The tendency of peripheral atherosclerosis to attract calcification and the problems inherent in luminal assessment in the presence of calcium
- □
The proximity of the distal femoral artery and the popliteal artery to adjacent bony structures, and the difficulties in employing “bone-removal” software features that may remove adjacent calcified vessel segments
- □
Accurate bolus tracking (ensuring that the acquisition speed does not exceed the contrast opacification)
Agreement of 16-slice MDCT with DSA is very good (96% sensitive), as is interobserver agreement (κ = 0.84–1.0). However, agreement varies considerably depending on the artery in question. Overestimation in a small percentage (2–3%) of cases occurs more frequently than does underestimation (1%). Anteroposteriorly directed narrowing and extensive calcification are common reasons for disagreement. Older scanners (4-MDCT) were inaccurate.
Confidence with MDCT is similar to that of MR angiography, but is less than with DSA, leading to more testing after CT angiography (35% of cases vs. 14% of cases after DSA).
More studies are needed with current scanners to understand the role of MDCT.
For images of peripheral artery disease, see Figures 27-4 through 27-9 .