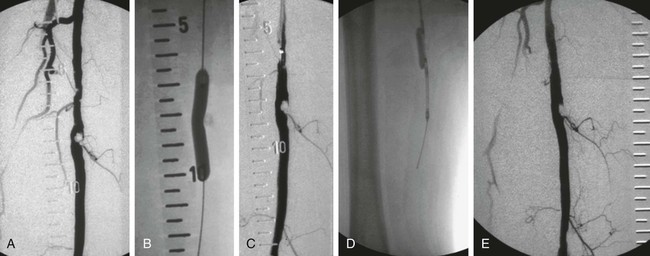
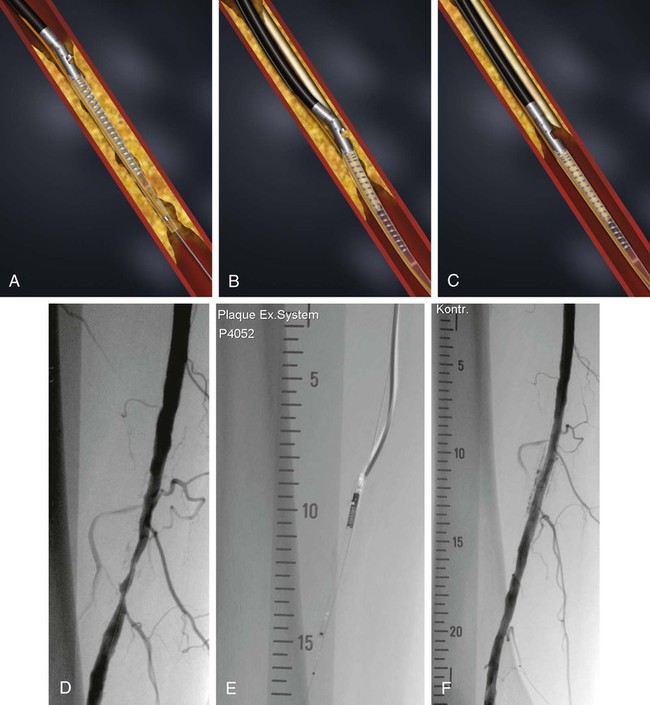
Balloon angioplasty of femoropopliteal lesions is limited by a low primary patency rate of 30% to 61% after 3 years, depending on lesion length and clinical stage.1 Even stent implantation or use of ablative methods (e.g., laser angioplasty) has not led to significant improvement in long-term results. Percutaneous removal of obstructive material via directional or rotational atherectomy represents a theoretical approach for reducing the rate of restenosis. However, atherectomy devices and other devices that mechanically or by other means remove plaque from clogged arteries have had limited efficacy and a spotty history of acceptance in coronary applications, where today they function as adjuncts to balloon angioplasty and stenting. At the beginning of 1990s, these devices were also used for the treatment of lower limb artery disease. They were bulky in terms of crossing profile and steerability (6F-14F; working diameter 3.5-9.7 mm). Most devices were not designed for an over-the-wire application (Fig. e9-1) before monorail technology became available. Atherectomy physically removes plaque by cutting, pulverizing, or shaving plaque in atherosclerotic arteries, using a mechanical catheter-deliverable endarterectomy device. Four U.S. Food and Drug Administration (FDA)-approved atherectomy devices are currently available for treatment of peripheral arterial disease (PAD): the SilverHawk Plaque Excision System (ev3/Covidien, Plymouth, Minn.), Orbital PAD System (Cardiovascular Systems Inc., St. Paul, Minn.), CVX-300 Excimer Laser (Spectranetics, Colorado Springs, Colo.), and Pathway PV Atherectomy System (Pathway Medical Technologies, Kirkland, Wash.). At present no specific recommendations are established for use of atherectomy devices in the treatment of lower limb arterial disease. Therefore, guidelines published by the American College of Cardiology should be followed. They indicate that atherectomy and stent placement are equivocal2: • Class I (Conditions for which there is evidence for and/or general agreement that a given procedure or treatment is beneficial, useful, and effective): 1. Endovascular procedures are indicated for individuals with a vocational or lifestyle-limiting disability due to intermittent claudication when clinical features suggest a reasonable likelihood of symptomatic improvement with endovascular intervention and (a) there has been an inadequate response to exercise or pharmacologic therapy and/or (b) there is a very favorable risk-benefit ratio (e.g., focal aortoiliac occlusive disease). (Level of Evidence: A [data derived from multiple randomized clinical trials or meta-analyses]) 2. Endovascular intervention is recommended as the preferred revascularization technique for Transatlantic Inter-Society Consensus type A (see Tables 20 and 21 and Figure 8 of the full-text guidelines) iliac and femoropopliteal arterial lesions. (Level of Evidence: B [data derived from a single randomized trial or nonrandomized studies]) 3. Translesional pressure gradients (with and without vasodilation) should be obtained to evaluate the significance of angiographic iliac arterial stenoses of 50% to 75% diameter before intervention. (Level of Evidence: C [only consensus opinion of experts, case studies, or standard of care]) • Class IIa (Conditions for which there is conflicting evidence and/or a divergence of opinion about the usefulness/efficacy of a procedure or treatment; weight of evidence/opinion is in favor of usefulness/efficacy): Stents (and other adjunctive techniques such as lasers, cutting balloons, atherectomy devices, and thermal devices) can be useful in the femoral, popliteal, and tibial arteries as salvage therapy for a suboptimal or failed result from balloon dilation (e.g., persistent translesional gradient, residual diameter stenosis greater than 50%, or flow-limiting dissection). (Level of Evidence: C) • Class IIb (Usefulness/efficacy is less well established by evidence/opinion.): 1. The effectiveness of stents, atherectomy, cutting balloons, thermal devices, and lasers for the treatment of femoral-popliteal arterial lesions (except to salvage a suboptimal result from balloon dilation) is not well established. (Level of Evidence: A) 2. The effectiveness of uncoated/uncovered stents, atherectomy, cutting balloons, thermal devices, and lasers for the treatment of infrapopliteal lesions (except to salvage a suboptimal result from balloon dilation) is not well established. (Level of Evidence: C) • Class III (Conditions for which there is evidence and/or general agreement that a procedure/treatment is not useful/effective and in some cases may be harmful.): 1. Endovascular intervention is not indicated if there is no significant pressure gradient across a stenosis despite flow augmentation with vasodilators. (Level of Evidence: C) 2. Primary stent placement is not recommended in the femoral, popliteal, or tibial arteries. (Level of Evidence: C) 3. Endovascular intervention is not indicated as prophylactic therapy in an asymptomatic patient with lower extremity PAD. (Level of Evidence: C) • Lower limb arterial (acute thrombotic occlusion) disease where interrupted blood flow would pose undue risk to thromboembolism • Lesions located in or distal to heavily calcified and/or tortuous vessels • Totally obstructed lower limb arteries where a guide wire cannot be advanced through the occlusion The Simpson AtheroCath (Devices for Vascular Intervention Inc. [DVI], Redwood City, Calif.) did not become well established for treatment of femoropopliteal lesions in the early 1990s because of its complex operation and poor results compared to balloon angioplasty. Design changes resulted in the Flexi-Cut Directional Debulking System (Guidant Corp., Indianapolis, Ind.). It is designed to enhance performance and ease of use. This system has a 134-cm working length, with a cutting window that contains an ultra-hard titanium nitrade cutter. The atraumatic tip has a cylindrical nosecone. The device is available in three sizes (for vessel sizes 2.5-2.9 mm, 3.0-3.4 mm, and 3.5-4.0 mm). Specific equipment needed for this system includes a drive unit, a rotating hemostatic valve, an 8F guiding catheter introducer, and a low-pressure inflation device for balloon inflation to fix the atherectomy chamber at the lesion site. When the chamber is fixed, the handheld motor drive can be activated. Usually at least two attempts are necessary after rotating the catheter in a 45-degree angle. An application in lower limb arteries is theoretically possible, but the outer diameter of the catheter has to match the vessel size (Fig. e9-2). A newly developed device with a slightly different operating mechanism is the SilverHawk Plaque Excision System (formerly from Foxhollow Corp., Redwood City, Calif. [now ev3/Covidien). It is an FDA-cleared device for the treatment of de novo and restenotic lesions in the peripheral arteries. The SilverHawk System uses a tiny rotating blade to shave away plaque from inside the artery. It consists of a flexible, smooth-coated shaft with a cutting unit on the distal end containing a rotating blade in a conical tapered housing (usually 6 cm in length) with a lateral window. As it is excised, plaque collects in the tip of the device and is then removed from the patient (Fig. e9-3). The SilverHawk Peripheral Catheter has an outer crossing profile from 0.088 to 0.105 inches (2.2-2.7 mm), with 0.014-inch monorail guidewire compatibility. The working length is available from 110 to 135 cm, and the tip length from 2.6 to 9 cm, with a variable capacity to collect up to 500 mg of plaque material. The proximal end of the catheter, which has a locking mechanism, connects to a disposable battery-operated electric motor. The device was designed for vessels measuring 3 to 7 mm in diameter. Additional catheters were subsequently introduced and incorporated design changes in the geometry and material used in the cutter to facilitate treatment of moderate to severely calcified lesions (TurboHawk). The latest so-called microefficient compression technology should allow up to 30% greater tissue collection. Whether the rotating stainless steel blade operating in directional atherectomy devices will cut through pure calcium is still unclear. Rotational atherectomy may also be used in heavily calcified lesions.2 The RotaLink Plus System (Boston Scientific Corp., Natick, Mass.) is a development of the former the Auth Rotablator (Heart Technology, Bellevue, Wash.). It was first introduced in 1998 in the United States for coronary arteries after receiving FDA approval. It is a flexible catheter-based device that uses a high-speed rotating burr covered with microscopic diamond crystals (Fig. e9-4). Rather than merely displacing atherosclerotic material as in balloon angioplasty, this high-speed rotational burr (up to 190,000 rpm) preferentially abrades inelastic plaque material while soft tissue and the vessel wall are forced aside. The RotaLink Plus System is a preconnected exchangeable handheld system and has a separate driving unit to activate the high-speed rotation of the burr (see Fig. e9-4). The available diameters of burr heads range from 1.25 to 2.5 mm, and the working length is 135 cm.
Atherectomy Devices
Clinical Relevance
Indications
Contraindications to Atherectomy
Equipment
Directional Atherectomy
Rotational Atherectomy
Atherectomy Devices