, Marilyn J. Siegel2, Tomasz Miszalski-Jamka3, 4 and Robert Pelberg1
(1)
The Christ Hospital Heart and Vascular Center of Greater Cincinnati, The Lindner Center for Research and Education, Cincinnati, OH, USA
(2)
Mallinckrodt Institute of Radiology, Washington University School of Medicine, St. Louis, Missouri, USA
(3)
Department of Clinical Radiology and Imaging Diagnostics, 4th Military Hospital, Wrocław, Poland
(4)
Center for Diagnosis Prevention and Telemedicine, John Paul II Hospital, Kraków, Poland
Abstract
The first attempt to repair a patient with transposition of the great arteries (TGA) was performed by Senning who successfully constructed an atrial baffle in 1958 [1]. The procedure employed an atrial baffle created from autologous tissue to redirect caval blood to the left atrium which emptied into the left ventricle which then pumped the deoxygenated blood to the lungs. Pulmonary venous blood was redirected to the morphologic right atrium and through the tricuspid valve into the right ventricle which then filled the aorta with oxygenated blood. Subsequently, in 1964, Mustard introduced an atrial switch procedure utilizing prosthetic patch material to create an intra-atrial baffle (Figs. 23.1 and 23.2) [2, 3]. See Fig. 19.22 for a diagram of the uncorrected circulation and the circulation after the atrial switch procedure which normalizes the blood flow circuit.
The first attempt to repair a patient with transposition of the great arteries (TGA) was performed by Senning who successfully constructed an atrial baffle in 1958 [1]. The procedure employed an atrial baffle created from autologous tissue to redirect caval blood to the left atrium which emptied into the left ventricle which then pumped the deoxygenated blood to the lungs. Pulmonary venous blood was redirected to the morphologic right atrium and through the tricuspid valve into the right ventricle which then filled the aorta with oxygenated blood. Subsequently, in 1964, Mustard introduced an atrial switch procedure utilizing prosthetic patch material to create an intra-atrial baffle (Figs. 23.1 and 23.2) [2, 3]. See Fig. 19.22 for a diagram of the uncorrected circulation and the circulation after the atrial switch procedure which normalizes the blood flow circuit.
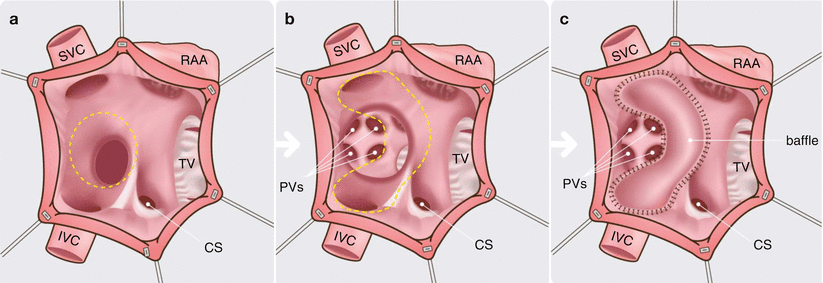
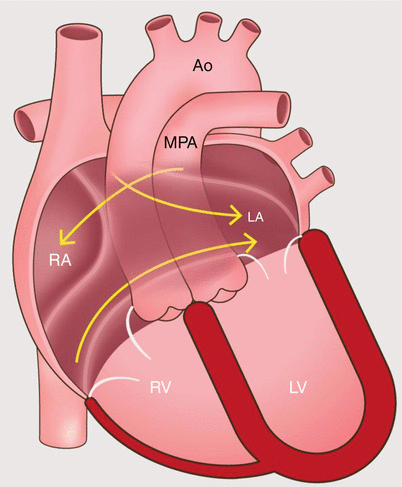

Fig. 23.1
An artist’s rendition of the Mustard operation. Panel (a) the right atrium is opened and the atrial septum is excised (dashed line). Panels (b) and (c) the interatrial baffle is sutured along the right atrium (dashed line) to redirect the systemic venous return to the left atrium, while the pulmonary venous return enters the right atrium. CS coronary sinus, IVC, inferior vena cava, PVs pulmonary veins, RAA right atrial appendage, SVC superior vena cava, TV tricuspid valve

Fig. 23.2
An artist’s diagram of the atrial baffle surgery in the D-transposition of the great arteries. Note the presence of ventriculo-arterial discordance with the origin of the main pulmonary artery from the left ventricle and the aorta from the right ventricle. To restore physiologic circulation, systemic blood return is redirected by a baffle to the left atrium, and pulmonary venous return enters the right atrium. Ao aorta, MPA main pulmonary artery, RA right atrium, RV right ventricle, LA left atrium, LV left ventricle
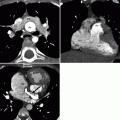
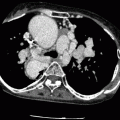
Both the Senning and Mustard procedures involve the redirection of blood flow at the atrial level. The morphologic left ventricle is the pulmonary ventricle and the morphologic right ventricle is the systemic ventricle which is subject to systemic loading pressures and subsequent compensatory hypertrophy. Although the atrial switch provides physiologic correction of the circulation, normal anatomic relationships of the aorta and pulmonary artery are not restored (Fig. 23.3). Examples of CT images of patients after the Mustard procedure are presented in Figs. 23.4 and 23.5.
Get Clinical Tree app for offline access

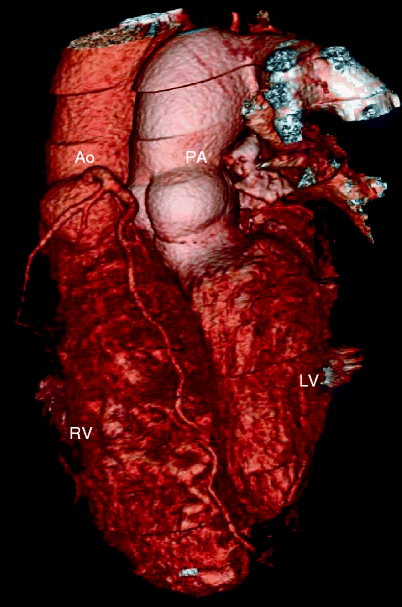
Fig. 23.3
Adult with D-transposition of great arteries and acute chest pain who had a Mustard procedure in infancy. A computed tomography scan was performed to evaluate for pulmonary embolus. This coronal 3D image demonstrates the pulmonary artery (PA) arising from the morphologic left ventricle (LV) and aorta (Ao) arising from the morphologic right ventricle (RV), typical of D-transposition. In the atrial switch, the correction is at the atrial level

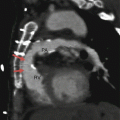
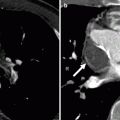
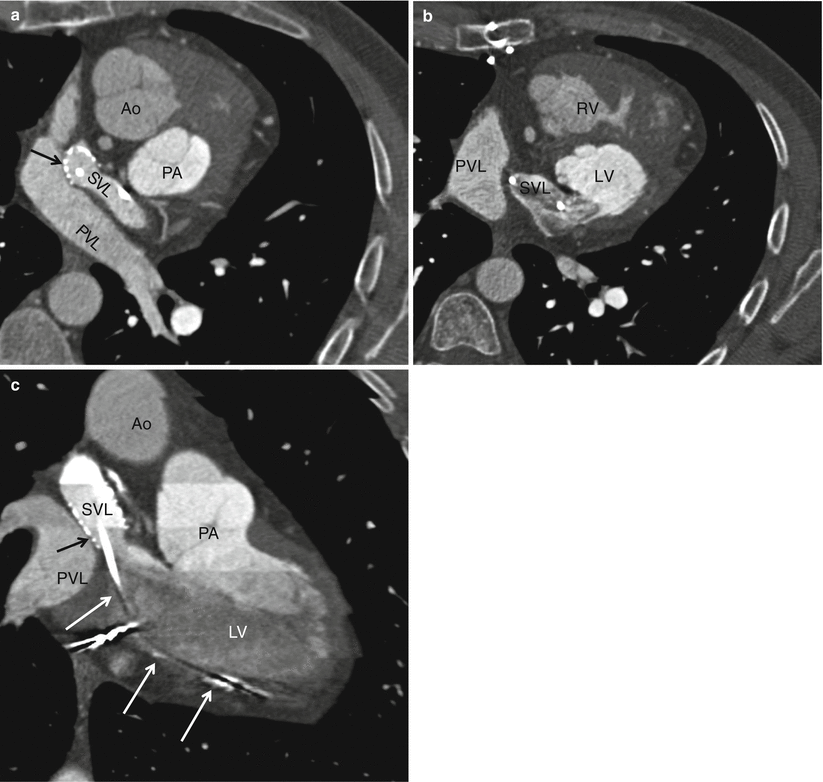
Fig. 23.4
A Mustard procedure in an adult who subsequently developed stenosis of the superior vena cava, requiring percutaneous stenting. Panel (a) is an axial view shows the systemic vein limb (SVL) (superior vena cava to left atrium) and pulmonary vein limb (PVL) (pulmonary veins to the right atrium) of the baffle. Panel (b) is an axial cut in a different plane clearly showing the emptying of deoxygenated blood from the SVL to the left ventricle. Panel (c) is a sagittal cut also demonstrating the ultimate connection of the SVL to the morphologic left ventricle. Pacemaker wires (best noted in panel c, arrows) are present in the baffle extending into the morphologic left ventricle (pulmonic ventricle). Complete atrioventricular block is common in transpositions due to marked elongation of the AV node. Also note the stent patency with inflow of contrast-enhanced blood from the superior vena cava above and noncontrast-enhanced blood from the inferior vena cava below to the systemic baffle limb panel (a and c). Ao aorta, PA pulmonary artery, LV




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree