CHAPTER 41 Atrial Septal Defect
Atrial septal defect (ASD) is one of the most commonly recognized congenital cardiac anomalies in adults. Knowledge of the embryologic development of the atrial septum provides the basis for understanding the pathophysiology, anatomy, and clinical manifestations of ASD. ASD is rarely diagnosed in infancy, and it even less commonly results in significant symptoms in infants. Patients, especially patients with small or isolated defects, are usually asymptomatic through the first 3 decades of life, although more than 70% become impaired by the fifth decade. Early surgical closure of most types of ASD is recommended.1
DEFINITION
An ASD is characterized by a defect in the interatrial septum that allows pulmonary venous return to pass from the left to the right atrium (i.e., left-to-right shunt), resulting in right atrial and right ventricular chamber dilation. There are four types of ASD. The first type is an ostium secundum defect. This type of defect is located in the area of the fossa ovalis and is the most common type of ASD, accounting for 75% of all cases. Although it usually consists of a single defect, fenestrated defects have also been reported. Secundum ASD can be associated with partial anomalous pulmonary venous return (<10%) and mitral valve prolapse. A second type of ASD is an ostium primum defect, which accounts for 15% of ASDs; this defect is located in the lower part of the interatrial septum and commonly involves other “endocardial cushion” defects. It can be associated with a “cleft” anterior mitral valve leaflet, which results in mitral regurgitation, or defects in the atrioventricular or membranous interventricular septum. The third type of ASD is a sinus venosus defect, which accounts for 10% of ASDs and involves the junction of the superior vena cava with the left atrium. Anomalous right upper lobe pulmonary venous return is a common manifestation with this type of ASD. The last type of ASD is a coronary sinus ASD, which is the rarest type and results from direct communication of the coronary sinus and the left atrium. Persistent left superior vena cava is commonly associated with coronary sinus ASD.1–4
PREVALENCE AND EPIDEMIOLOGY
ASD accounts for 10% of all congenital heart disease, and for 22% to 40% of congenital heart disease in adults. ASD is twice as common in girls as in boys. Most ASDs occur sporadically as a result of spontaneous genetic mutations; however, hereditary forms have been reported. Associated extracardiac congenital defects are present in 25% of infants, and about 30% have an ASD in association with a hereditary syndrome, such as Down syndrome, Holt-Oram syndrome, and Noonan syndrome.2
ETIOLOGY AND PATHOPHYSIOLOGY
Ostium secundum ASD results from incomplete adhesion between the original ridge of tissue of the valve of the foramen ovale and the septum secundum. The patent foramen ovale usually results from abnormal resorption of the septum primum during the formation of the foramen secundum. Resorption in abnormal locations causes a fenestrated or netlike septum primum. An abnormally large foramen ovale can occur as a result of defective development of the septum secundum. The normal septum primum does not close this type of abnormal foramen ovale at birth. A combination of excessive resorption of the septum primum and a large foramen ovale produces a large ostium secundum ASD.1,2
Ostium primum ASD is caused by incomplete fusion of septum primum with the endocardial cushion. The defect lies immediately adjacent to the atrioventricular valves, either of which may be deformed and incompetent. In most cases, only the anterior leaflet of the mitral valve is displaced, and it is commonly cleft. The tricuspid valve is usually not involved. The defect is often large, and a “complete” atrioventricular canal defect may occur with an ASD associated with a ventricular septal defect.1,2
Sinus venosus ASD occurs when abnormal fusion between the embryologic sinus venosus and the atrium occurs. These defects generally lie high in the atrial septum near the entry of the superior vena cava, and are generally associated with anomalous right upper pulmonary venous return. An uncommon inferior type is associated with partial anomalous return of the right lower pulmonary vein. Anomalous drainage can be into the right atrium, the superior vena cava, or the inferior vena cava.1,2
Coronary sinus ASD is characterized by an unroofed coronary sinus and persistent left superior vena cava that drains into the left atrium. A dilated coronary sinus often suggests this defect. The diagnosis can be made explicitly by MRI, CT, echocardiography, or angiography by injecting contrast agent into the left upper extremity. Coronary sinus opacification, which precedes right atrial opacification, confirms the diagnosis.1,2
A small ASD results in trivial shunting and has no hemodynamic consequences. Larger defects are associated with substantial shunting, which may lead to volume overload of the right atrium, right ventricle, and pulmonary arteries. The magnitude of left-to-right shunting depends on the size of the ASD, the relative compliance of the two ventricles, and the pulmonary and systemic vascular resistance. If left untreated, the left-to-right shunting may result in pulmonary hypertension, right ventricular failure, decreased right ventricular compliance, and potentially right-to-left shunting (Eisenmenger syndrome). Eisenmenger syndrome secondary to ASD is rare in adults, however, occurring in about 5% of patients.1,2
MANIFESTATIONS OF DISEASE
Clinical Presentation
ASD generally goes unnoticed because patients are usually asymptomatic in the first and second decades of life. The most common manifestations of this condition are development of fatigue, dyspnea on exertion, and exercise intolerance generally during the third and fourth decades. The most common presenting symptom is dyspnea and easy fatigability. Other symptoms include palpitations, syncope, and heart failure. The development of palpitations related to supraventricular arrhythmia is the most common symptom in adults. Occasionally, patients may present with paradoxical embolization or recurrent respiratory infection. If left untreated, patients with hemodynamically significant ASD develop symptoms of right-sided heart failure, including jugular venous distention and lower extremity edema. Over time, pulmonary pressure increases because of increased pulmonary resistance, and the shunt may reverse with predominant right-to-left shunting (Eisenmenger syndrome).3,4
The cardiac examination is generally characterized by signs of right heart overload. Cardiac auscultation reveals a normal S1, fixed splitting of S2, and a systolic outflow murmur as a result of the increased flow into the main pulmonary artery. Shunting across the ASD does not produce a murmur. In patients with ostium primum ASD and a cleft anterior mitral valve leaflet, a mitral regurgitation murmur can be appreciated at the apex. The development of pulmonary hypertension results in narrowing of the splitting of S2 and accentuation of the pulmonary closure component. The pulmonic systolic murmur decreases in intensity, and a diastolic pulmonic regurgitation murmur may appear. The development of Eisenmenger syndrome results in cyanosis and clubbing.3,4
Imaging Indications and Algorithm
If echocardiography does not provide adequate assessment of the ASD, CT may be performed; however, MRI is better at assessing ASD because it allows for quantitative assessment of shunts and determination of shunt fractions. Less commonly used modalities for the assessment of ASDs are nuclear medicine and angiography, which provide less anatomic information than the above-mentioned modalities, but yield quantitative assessment of shunt fractions and hemodynamic pressure measurements in the case of invasive angiography. The algorithm for the work-up generally can be accomplished with echocardiography, but rarely, other imaging modalities, most commonly MRI or angiography, can be applied if discrepancies exist.5
Imaging Technique and Findings
Radiography
A plain chest radiograph (Fig. 41-1) is characterized by increased caliber of the main pulmonary artery segment and the hilar and parenchymal pulmonary arteries (i.e., shunt vascularity). Until pulmonary resistance increases, resulting in pulmonary hypertension, the parenchymal vessels extend farther toward the pleura than expected, and appear to taper normally. The pulmonary vessels are sharp. The left-to-right shunt volume loads the right heart, resulting in right atrial and ventricular dilation and clockwise (leftward) cardiac rotation. Increased pulmonary venous return is decompressed across the defect in the interatrial septum; the left atrium and ventricle are normal in size (Fig. 41-2). The typical chest film of a patient with ASD is shunt vascularity with right heart enlargement and a normal left heart.4
When pulmonary resistance increases, causing pulmonary hypertension, the appearance of the chest film changes (Fig. 41-3). The central (extrahilar) pulmonary arteries remain enlarged, but the parenchymal pulmonary artery segments become vasoconstricted, producing the typical appearance of acute change of pulmonary artery caliber seen in pulmonary hypertension. Careful evaluation of the peripheral pulmonary artery segments reveals extension toward the pleura beyond that found in a normal examination, but in smaller caliber vessels. As long as the shunt is left-to-right, right heart enlargement and a normal left heart are seen. In the rare circumstance of shunt reversal, progressive right heart decompression and left heart enlargement may be seen.4,5
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


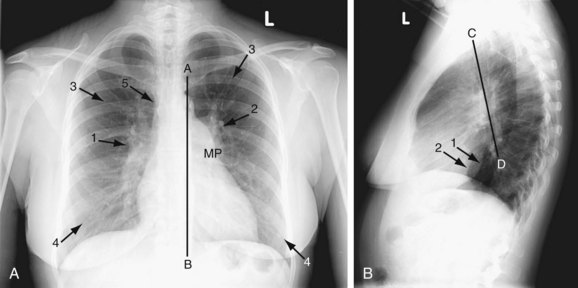
 FIGURE 41-1
FIGURE 41-1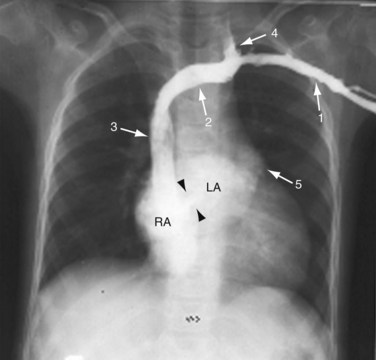
 FIGURE 41-2
FIGURE 41-2