and Ashutosh Dash2
(1)
Nuclear Security and Isotope Division, Oak Ridge National Laboratory, OAK RIDGE, USA
(2)
Isotope Production and Applications Division, Bhabha Atomic Research Centre, Mumbai, India
8.1 Introduction
Because of the very high LET and opportunity to sterilize target cells without the potential irradiation and damage of nontarget adjacent cells which is encountered with the use of beta-emitting radioisotopes, interest in the use of alpha emitters, especially for oncology applications, has dramatically increased in the last decade. Very eloquent chemistry and innovation production strategies have provided access to adequate activity levels of many of these desired radioisotopes for the first time. Table 8.1 provides properties of representative radionuclides in various decay chains which provide alpha-emitting radioisotopes of interest for targeted alpha therapy (TAT) key information for key alpha-emitting radioisotopes of current and expected interest. Chapter 2 introduces and provides key examples of the biological strategies for targeting alpha emitters, and Chaps. 6 and 7 provide introduction to the use of both research reactors and accelerators for production of these species, and Chap. 7 discusses radionuclide generator systems which provide several alpha emitters of interest. This chapter is dedicated to a discussion of the processing and purification chemistry which are required to obtain these radioisotopes for subsequent radiolabeling and biological investigation.
Table 8.1
Radioactive properties of representative radionuclides in decay chains which provide alpha-emitting radioisotopes of interest for targeted alpha therapy (TAT)a
Radioisotope | Half-life | Decay mode | Radiation energy | Comment | ||
|---|---|---|---|---|---|---|
Alpha MeV | Beta MeV | Gamma keV | ||||
Actinium radioisotopes | ||||||
Actinium-225 | 10 days | α | 5.935 (100 %) | 99.8 | Parent of 213Bi | |
Actinium-227 | 21.77 years | α | 5.042 (1.38 %) | 0.0449 (98.6 %) | 100 | Parent of 227Th/223Ra |
Bismuth radioisotopes | ||||||
Bismuth-212 | 61 m | α, β− | 6.207 (35.9 %) | 2.254 (64 %) | 238, etc. | |
Bismuth-213 | 45 m | α, β− | 5.982 (2.09 %) | 1.427 (97.91 %) | 440 | |
Lead radioisotopes | ||||||
Lead-212 | 10.6 h | β− | 0.574 (100 %) | 0.15 | Parent of 212Bi | |
Radium radioisotopes | ||||||
Radium-223 | 11.43 days | α | 5.979 (100 %) | 269, etc. | 223RaCl2 = Xofigo® | |
Radium-224 | 3.66 days | α | 5.788 (100 %) | 240, etc. | Parent of 212P | |
Radium-226 | 1,600 years | α | 4.870 (100 %) | 186 | Important target material | |
Thorium radioisotopes | ||||||
Thorium-227 | 18.65 days | α | 6.146 (100 %) | 236, etc. | Parent of 223Ra | |
Thorium-228 | 1.912 years | α | 5.520 (100 %) | 216, etc. | Parent of 224Ra | |
Thorium-229 | 7.34 × 103 years | α | 5.187 (100 %) | 193, etc. | Parent of 225Ac | |
Thorium-232 | 1.405 × 1010 years | α | 4.082 (100 %) | 59 | Parent of 228Th, 224Ra | |
8.2 Production and Processing of Alpha Emitters in the Thorium Series
8.2.1 Actinium-225
Actinium-225 (T 1/2 = 10.0 days) can be obtained from decay of 229Th (Fig. 8.1) and decays by emission of three alpha-particles through the 221Fr (half-life, 4.8 min)/217 at (half-life, 32.3 ms)/213Bi (half-life, 45.6 min) decay chain. There are several avenues to obtain 299Th which include neutron irradiation of 232Th which leads to 233U, from which α-decay forms 229Th, with subsequent α-decay to 225Ac. Another strategy is reactor irradiation of 226Ra, by multiple neutron captures and also provides 229Th (see Chap. 3). Because of its very long 7340-year half-life, 229Th represents a convenient source material to obtain 225Ac on a regular basis by elution of the 229Th/225Ac generator. In addition, 225Ac has been directly produced via 800 MeV high-energy irradiation of natural thorium (232Th) targets (Zhuikov et al. 2012). Actinium-225 is being evaluated for in vivo therapeutic applications because of its useful half-life of 10 days and emission of multiple α-particles. In addition, the 225Ac/213Bi generator provides the short-lived (half-life 45 m in) 213Bi alpha emitter which has been widely studied for targeted alpha therapy (see Chap. 3).
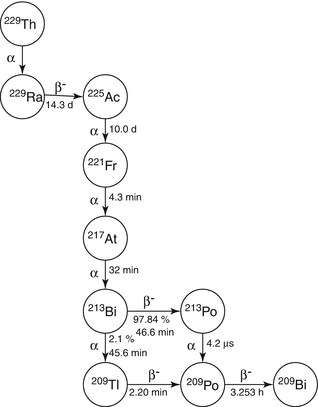

Fig. 8.1
The 225Ac and 213Bi decay chain. The 225Ac is obtained as the decay product of 229Th and decays by α-emission through 221Fr, 217At, and 213Bi, each of which also emits an α-particle
There are several production strategies to provide 225Ac from 226Ra which include irradiation in a nuclear reactor where there is successive multiple neutron capture on 226Ra. A mixture of 227Ac, 228Th, and 229Th is produced (Boll et al. 1997), from which 229Th can be directly obtained with a relatively high yield and 225Ac can be isolated by ion exchange separation method using titanium phosphate adsorbent. Another promising production pathway is via the 226Ra(p,2n)225Ac proton-induced reaction using 18 MeV protons which requires repeated irradiation of 226Ra. After production, 225Ac requires purification on a Dowex-50 column (Geerlings et al. 1993) before use for radiolabeling or for fabrication of the 225Ac/213Bi generator. A more recent strategy involves the 200 MeV high-energy spallation of natural thorium targets (232Th) (Weidner et al. 2012). The preliminary cross section data have suggested that multi-Curie activity levels of both 225Ac and 223Ra can be produced using accelerators available at both BNL and LANL. Other routes which have been proposed but evidently not yet evaluated include the use of alpha-particles for 229Th production by the 226Ra(3He,γ)229Th and 226Ra(α,n)229Th reactions (Mirzadeh and Garland 2010).
Production of 227Ac is also possible by the 226Ra + γ → 225Ra + n photonuclear reaction. This reaction is virtually instantaneous, so that 225Ra activity levels reach a maximum value after irradiation and will decrease slowly over time (Melville et al. 2006). Since there is no direct production avenue to provide 225Ac from the irradiation of 226Ra, the natural decay of 225Ra is utilized where 225Ra subsequently decays to 225Ac by beta emission (T 1/2 = 14.8 days) by the 225Ra 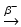 225Ac decay reaction.
225Ac decay reaction.
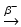 225Ac decay reaction.
225Ac decay reaction.The therapeutic use of the actinium alpha emitters provides greater cytotoxicity than use of 213Bi-labeled agents since the 225Ac 10.0 days half-life and emission of 4 alpha-particles from decay of 227Ac, as demonstrated in the prolonged survival of mouse xenograft models for several types of experimental cancers (McDevitt et al. 2001). A major expected impediment of using 225Ac for many therapeutic strategies is that the chelation binding energy is not strong enough to withstand the recoil energy released during alpha-particle emission of the actinium ion (between 100 and 200 keV). This energy usually release the 225Ac from chelate stabilization sphere (Davis et al. 1999; Kennel and Mirzadeh 1998; Deal et al. 1999). In this manner, the 225Ac daughter radionuclides are released from the localized site selectivity and diffuse away, causing cell damage in normal tissue (McDevitt et al. 1998; Schwartz et al. 2011). However, methods for stabilization of the 225Ac to recoil release of decay products have been evaluated, and simple gold-coated nanoparticles have exhibited stability in animal studies (McLaughlin et al. 2013).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree