BENIGN AND MALIGNANT EPITHELIAL TUMORS: LESIONS OF GLANDULAR ORIGIN
Key Points
- Epithelial tumors of glandular most commonly arise from the major and minor salivary glands.
- Benign and malignant glandular tumors typically have distinct yet not strictly diagnostic gross morphology as seen on computed tomography and magnetic resonance imaging.
- These tumors have a great deal of biologic variability.
This chapter takes a pathologic point of view of tumors of glandular cell origin. These may arise from mucosal and skin sources, but those are considered in conjunction with epithelial and cutaneous tumors in Chapters 23 and 24. The tumors considered here arise from glands. Most, aside from thyroid adenomas, arise from the major salivary and lacrimal glands. Some arise from the ubiquitous minor salivary glands scattered throughout the extracranial head and neck. Each tumor type is considered in terms of its occurrence at various head and neck sites. Also, the particular effect of a neoplasm’s spread pattern, natural history, and cellular structure have on its computed tomography (CT), magnetic resonance (MR), and ultrasound (US) anatomic appearance1 are summarized since these general trends are presented in Chapter 21 in significant detail. The physiology as reflected on metabolic imaging studies such as fluorine-18 2-fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) in this group of tumors is not predictable except that the more aggressive the lesion, the more likely it is to be FDG avid.2 Unfortunately, this leads to false-negative PET studies in malignant tumors, limiting the reliability and, therefore, usefulness of FDG-PET in the diagnosis and management of this group of neoplasms in an unpredictable manner. Diffusion-weighted magnetic resonance imaging (MRI) may be a tool useful to differentiate benign and malignant glandular neoplasms.3,4 While this is true in some cases, it is not useful for definitive clinical decision making and is certainly not a substitute for biopsy. The clinical and imaging implications of a particular type of tumor at a specific anatomic location are presented in site-specific discussions later in the text.
When evaluating the malignant varieties of glandular tumors, the appearance natural history of the lesion must be understood and appreciated on imaging with regard to its local extent, potential for perineural spread, and spread to regional lymph nodes. These are issues discussed elsewhere in general in Chapter 21 and in discussions of specific sites of occurrence.
BENIGN TUMORS OF GLANDULAR ORIGIN
Adenomas
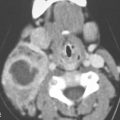
Adenomas most commonly arise from salivary and endocrine glandular tissue in the head and neck. Pleomorphic adenomas of the parotid gland (Figs. 22.1 and 22.2) and thyroid adenomas are the types that present for imaging most commonly as a mass and are also the most commonly imaged as incidental findings (Fig. 22.3). Thyroid and parathyroid adenomas are discussed in more detail in Chapters 170 and 174. Invasive pituitary adenomas are discussed in Chapter 32. This chapter deals primarily with tumors of salivary gland origin.
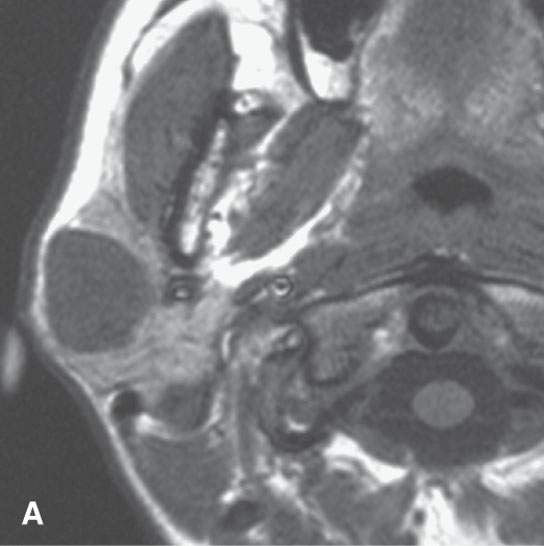
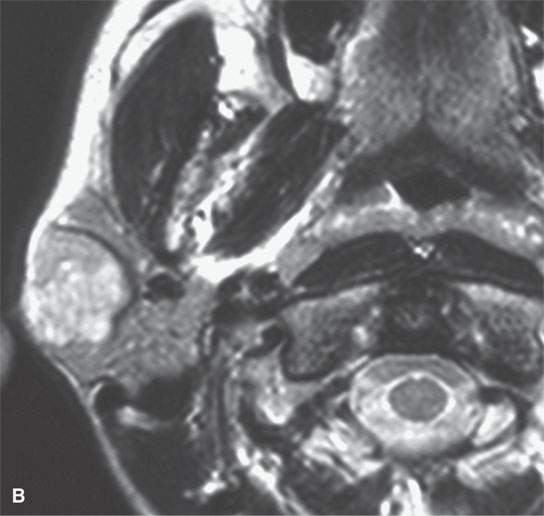
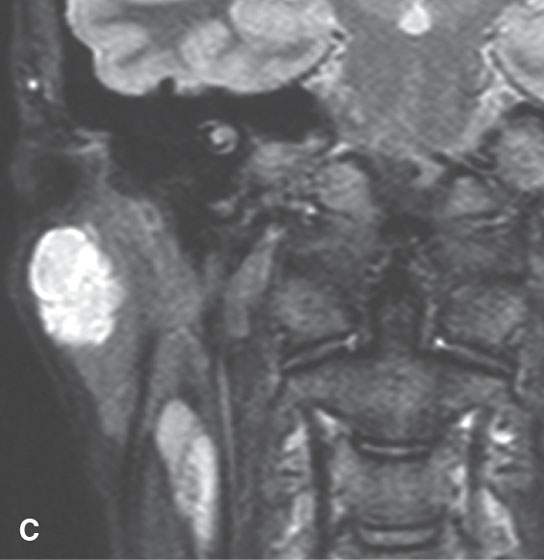
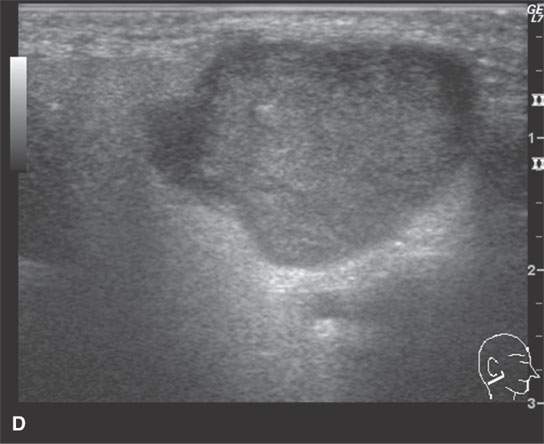
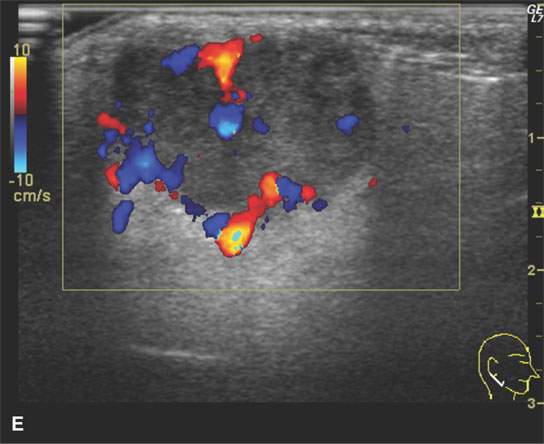
FIGURE 22.1. Magnetic resonance imaging and ultrasound (US) of the same benign mixed tumor of the parotid gland. A: Non–contrast-enhanced T1-weighted image. B: T2-weighted image. C: Short T1 inversion recovery (STIR) image. D: US showing good through transmission and a strong back wall reflecting the microcystic nature of the lesion. E: Color flow US showing a scattered peripheral and nonspecific flow pattern not consistent with a vascular malformation.
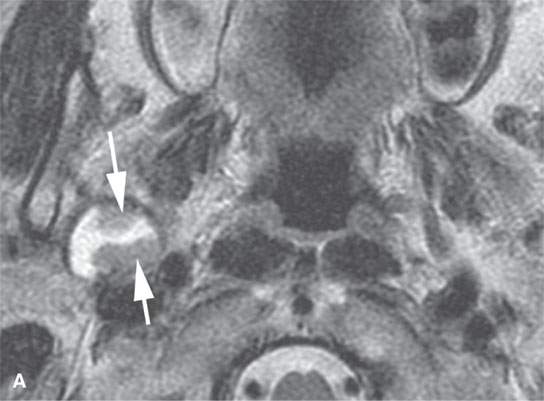
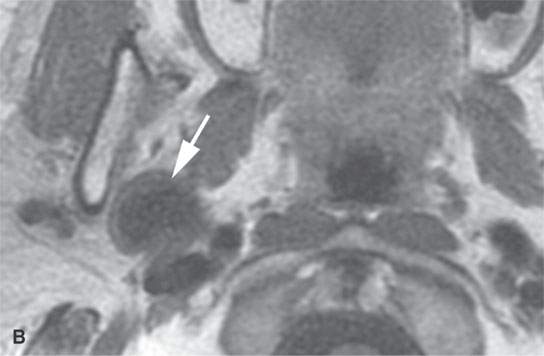
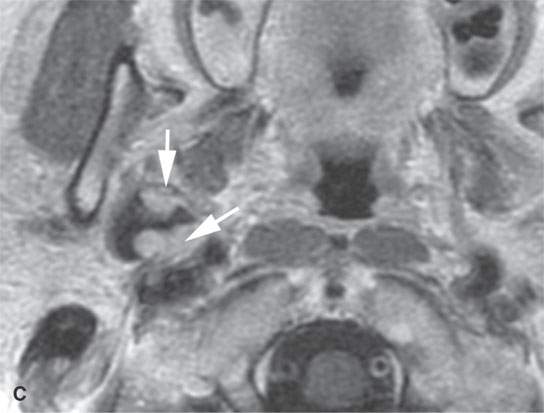
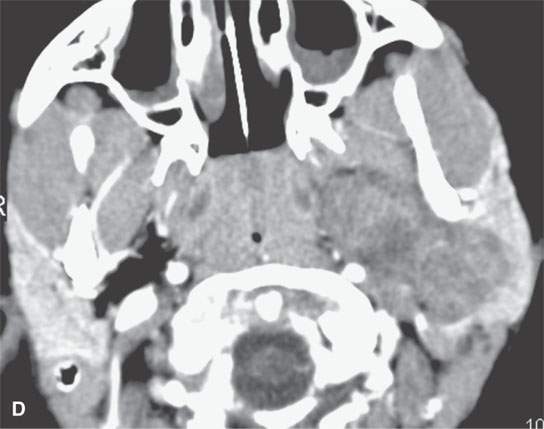
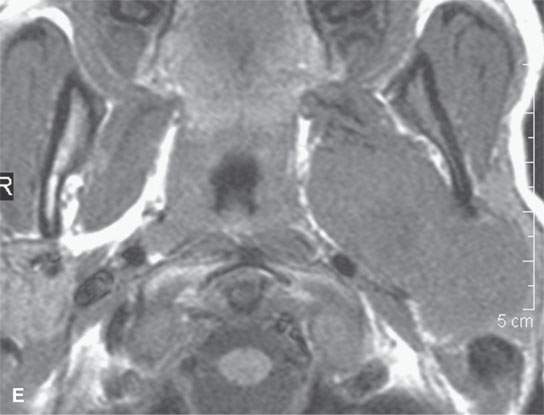
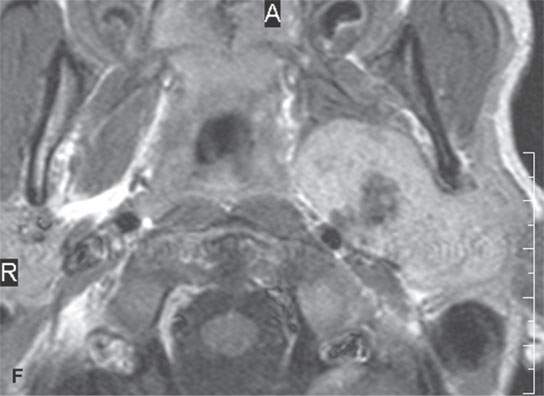
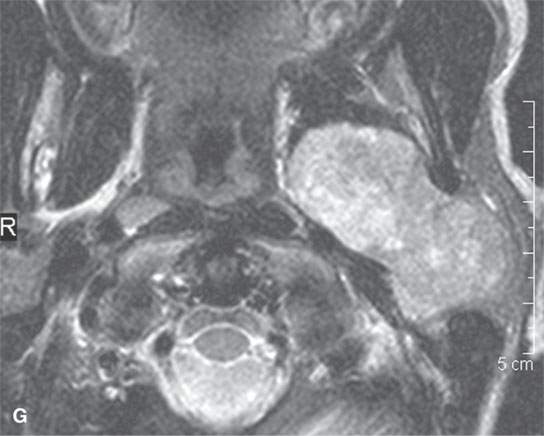
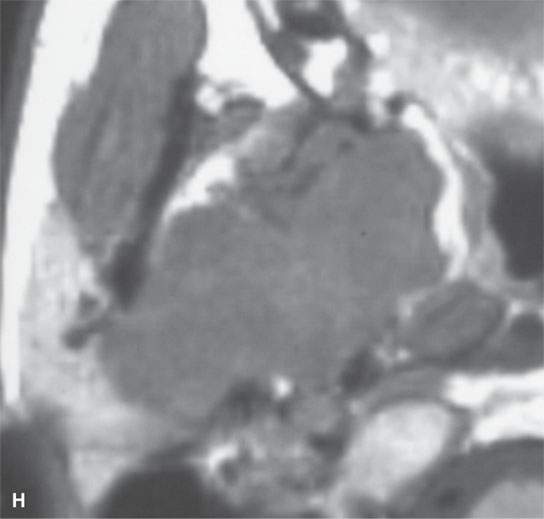
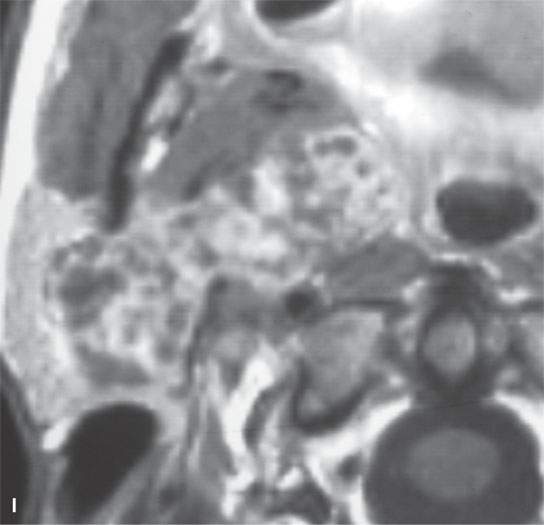
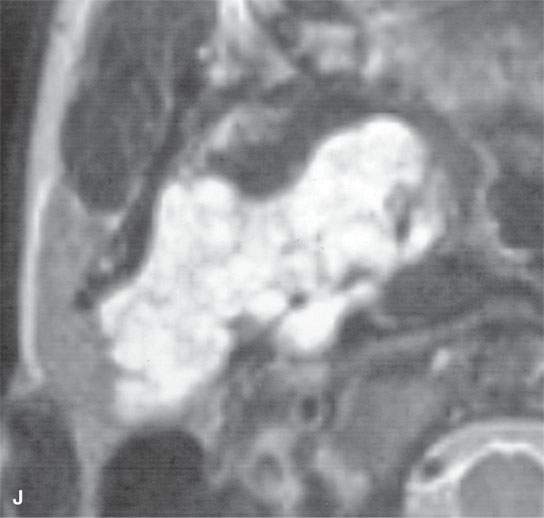
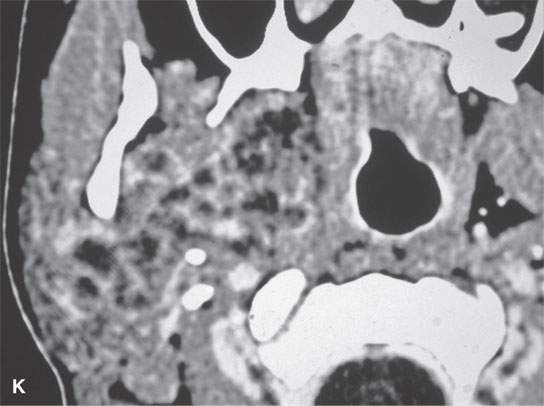
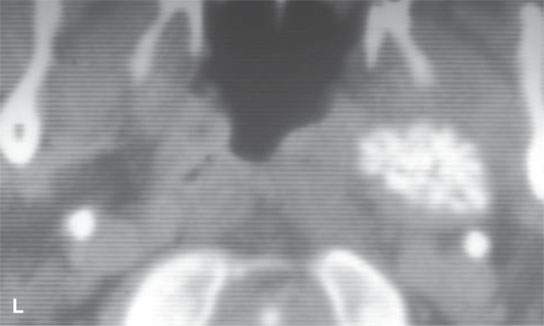
FIGURE 22.2. Magnetic resonance imaging and computed tomography (CT) studies of four benign mixed tumors of the parapharyngeal space demonstrating the range of appearance possible in these relatively common parapharyngeal lesions. The first tumor was discovered incidentally. The microcystic or macrocystic zones are admixed with solid cellular components (arrows) in a T2-weighted (T2W) image (A), non–contrast-enhanced T1-weighted (T1W) image (B), and contrast-enhanced T1W image (C). The second tumor is predominantly solid and cellular: contrast-enhanced computed tomography (CECT) (D), non–contrast-enhanced T1W image (E), contrast-enhanced T1W image (F), and T2W image (G). The third tumor is composed of many macro- and microcystic components: non–contrast-enhanced T1W image (H), contrast-enhanced T1W image (I), T2W image (J), and CECT (K). L: CT of a benign mixed tumor with extensive calcification a highly unusual appearance. When calcification occurs in these lesions, it is usually focal or scattered multifocal in nature.
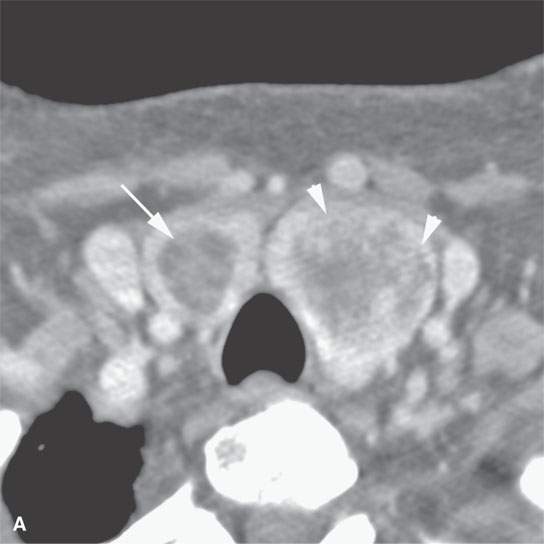
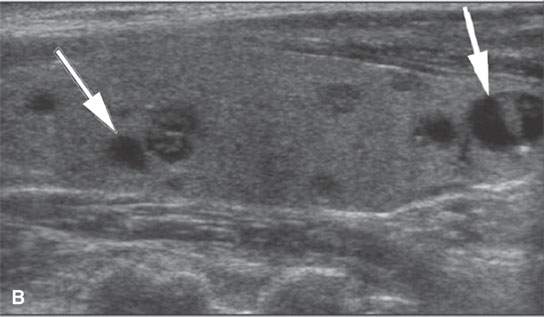
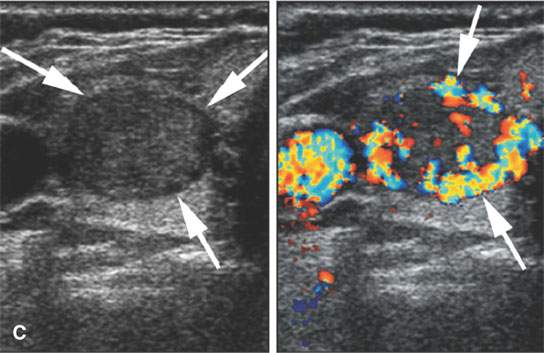
FIGURE 22.3. Three patients with thyroid adenomas. A: Contrast-enhanced computed tomography shows incidentally imaged adenomas—-the one in the right lobe sharply marginated (arrow) and the one in the left lobe slightly less so (arrowheads); both are benign. B: Ultrasound (US) of multiple small adenomas, the larger of which (arrows) are not obviously cystic. C: Routine US shows a halo (arrows), a fairly reliable sign of the lesion being benign and color flow US showing obvious hypervascularity (arrows), which is common in both benign and malignant thyroid nodules.
Pleomorphic adenomas are most frequently encountered in the parotid gland and prestyloid parapharyngeal space (Figs. 22.1, 22.2, and 22.4). Sites of origin that also commonly come to imaging are the submandibular gland (Fig. 22.5), oral cavity/buccal space (Fig. 22.6), and hard palate. Sites of very infrequent presentation include the sublingual space in the floor of the mouth, neck (Fig. 22.7), and larynx and trachea (Fig. 22.8).
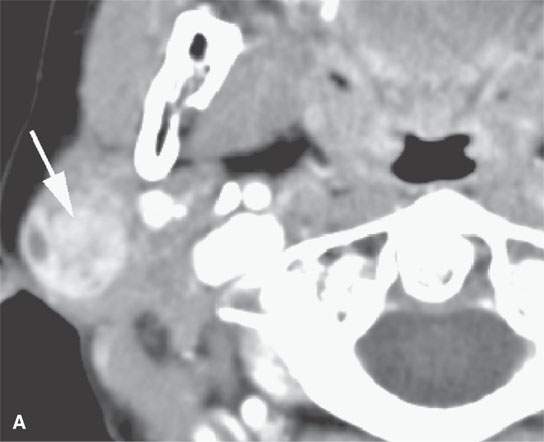
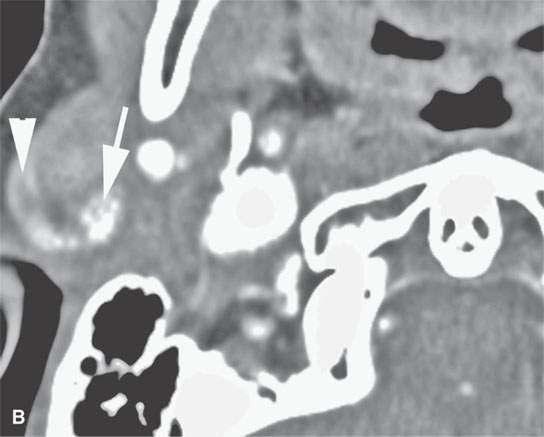
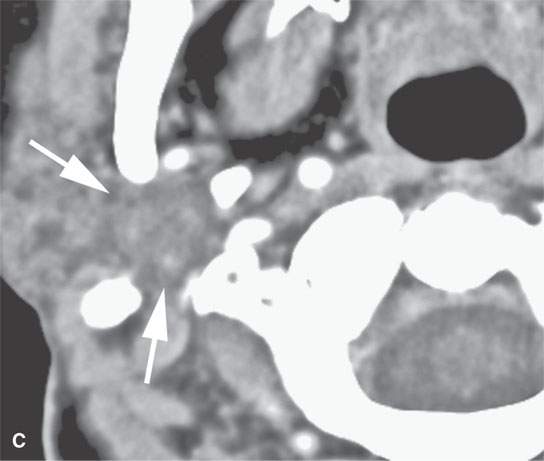
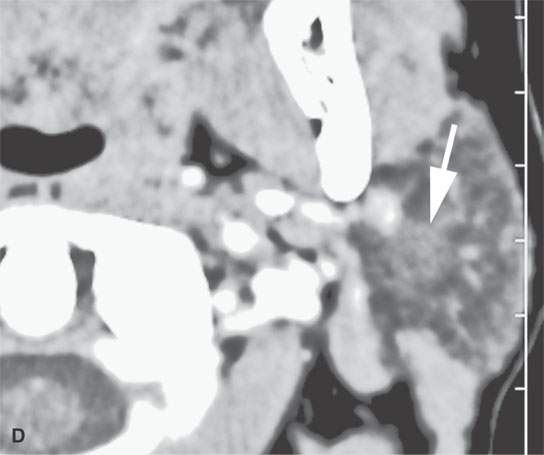
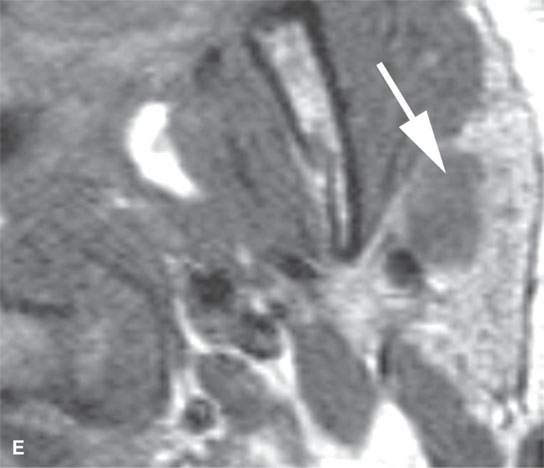
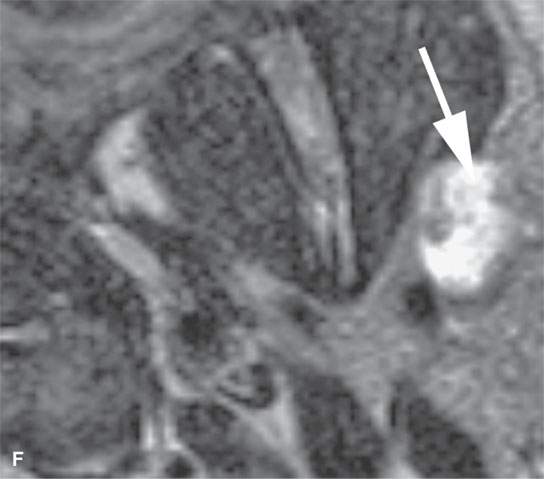
FIGURE 22.4. A–D: Contrast-enhanced computed tomography of four benign mixed tumors of the parotid gland: one that generally enhances (arrow in A), one that is partially calcified (arrow) and only minimally enhancing (B), one in the deep portion of the gland that is difficult to discern (arrow in C), and one that does not enhance at all and might not be seen if the gland were not fatty (arrow in D). E, F: T1- and T2-weighted images show the tendency of parotid benign mixed tumors to be more conspicuous on magnetic resonance imaging.
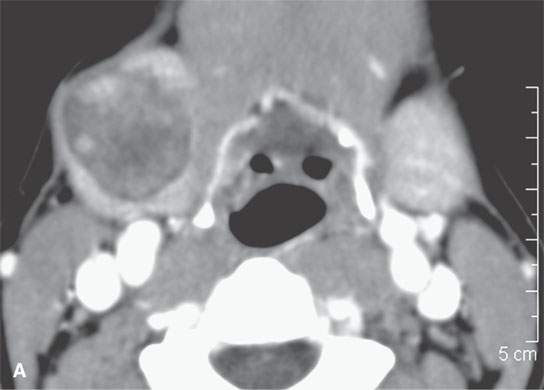
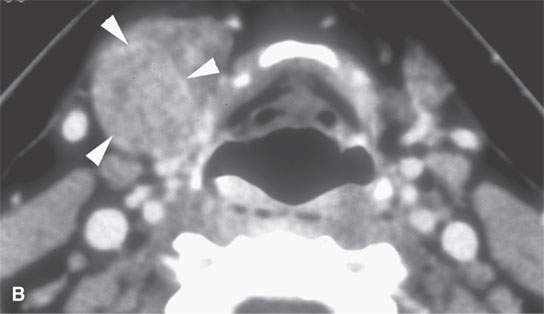
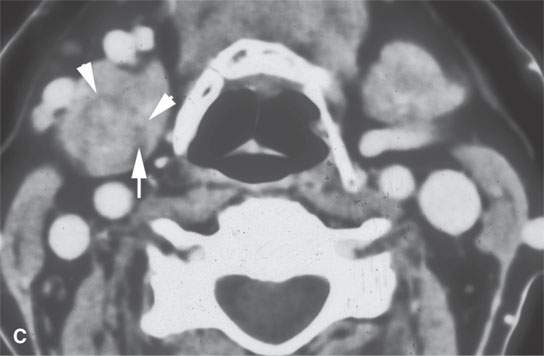
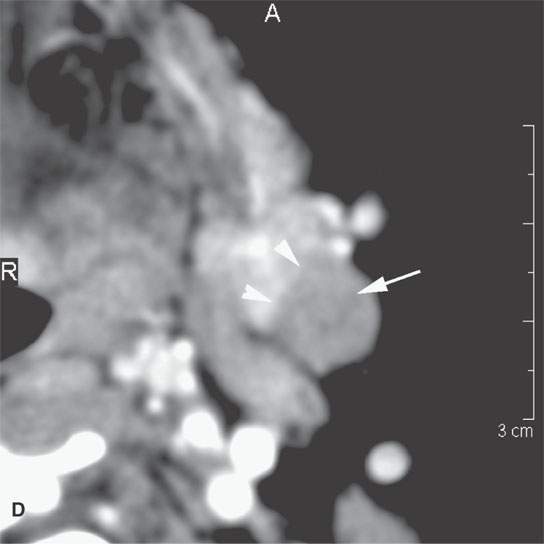
FIGURE 22.5. A–D: Contrast-enhanced computed tomography of four benign mixed tumors of the submandibular: one that is relatively low density (A), one that is minimally enhancing (arrowheads in B), one that is sharply marginated (arrowheads in C) and but for its peripheral lucency would be difficult to tell from the rest of the gland parenchyma (arrow), and one that does not enhance but is sharply marginated (arrowheads in D); the gland does enhance, and tumor protrudes from the gland surface (arrow).
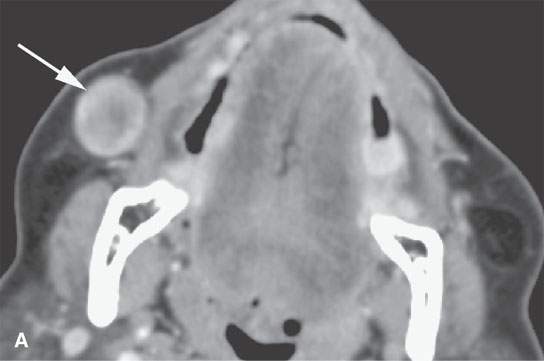
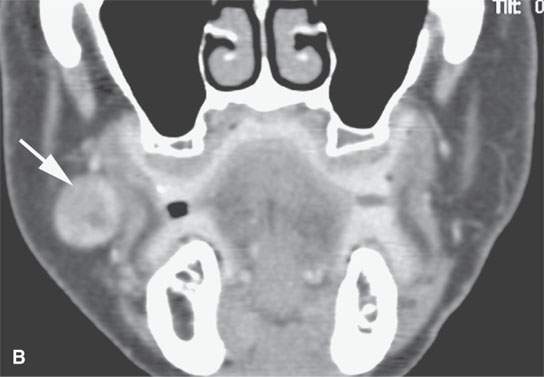
FIGURE 22.6. Contrast-enhanced computed tomography of a benign mixed tumor of the buccal space (arrows).
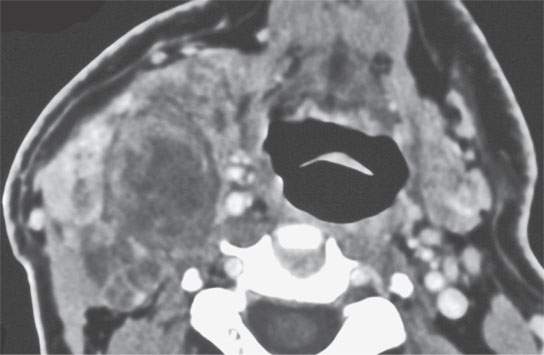
FIGURE 22.7. Contrast-enhanced computed tomography of a benign mixed tumor of the lateral compartment of the neck.
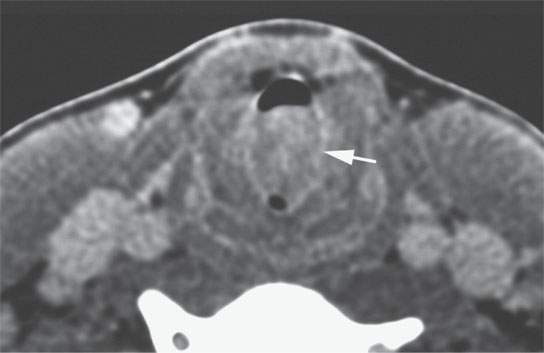
FIGURE 22.8. Contrast-enhanced computed tomography of a benign mixed tumor of the larynx and trachea.
Rests of ectopic salivary tissue can be found in the tonsils, lymph nodes, larynx, hypopharynx, neck, external auditory canal, middle ear, and thyroglossal duct; pleomorphic adenomas, therefore, may occur in these sites.5,6 Pleomorphic adenomas present more commonly in females, and peak age incidence is about 50 years and older.
The margins of benign mixed tumors are typically well demarcated from surrounding tissue, are smooth, and have a capsule of variable thickness (Figs. 22.1, 22.2, and 22.4–22.8). They often are lobulated, which on sectional imaging can lead to the mistaken impression that they are multifocal. Compression of surrounding tissue is common, but invasion of these tissues is not common despite indistinct tumor margins that may suggest such locally aggressive behavior (Fig. 22.7). Attempts to enucleate these tumors usually leave foci of tumor in the operative bed probably because of small protrusions of tumor through the thinner portions of the tumor-pseudocapsule. Multifocal recurrence following attempted enucleation is, therefore, commonplace (Fig. 22.9). Such recurrence is frequently admixed with scar and, if in the parotid gland, may surround the facial nerve, making surgical resection potentially very morbid due to potential facial nerve injury. The pseudocapsules are often not visible on CT but may be seen on T2-weighted MR (Fig. 22.2A), if they are thick and fibrous enough, to appear different from surrounding compressed glandular or soft tissue elements.6
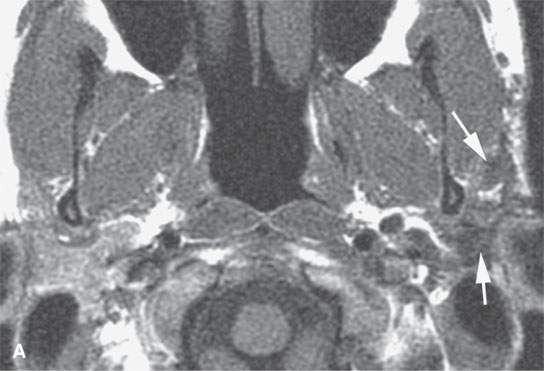
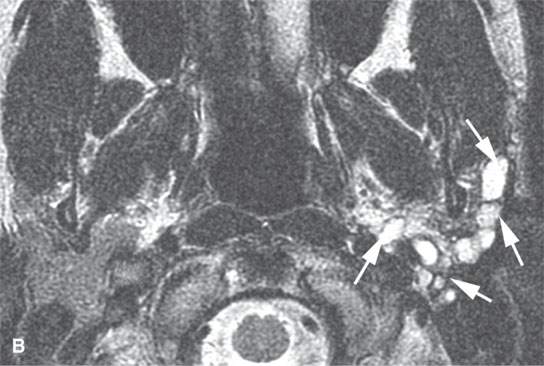
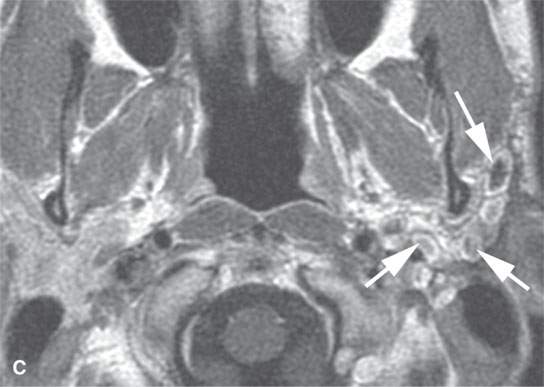
FIGURE 22.9. Magnetic resonance imaging of a patient with a persistent benign mixed tumor as a result of tumor spillage during the initial attempt at removal. The recurrent nodules (arrows) are admixed with scar and present all along and deep to the expected course of the main trunk of the facial nerve: non–contrast-enhanced T1-weighted image (A), T2-weighted image (B), contrast-enhanced T1-weighted image (C).
The internal morphology of mixed tumors may be homogeneous or very complex (Figs. 22.1, 22.2, and 22.4–22.8). Both epithelial and mesenchymal elements must be present for pathologic diagnosis and to distinguish them from the far less common monomorphic adenoma (Fig. 22.10). The stroma may be scant or abundant relative to the epithelial component, leading to a diverse MR and CT appearance. The stroma may be mucoid, fibroid, chondroid, myxochondroid, and/or vascular. Calcification and ossification may occur but are uncommon (Figs. 22.2L and 22.4A). On CT, the tumors may show areas of marked contrast enhancement, low density (fluidlike), muscle density, and calcification (Figs. 22.2 and 22.4–22.8). On non–contrast-enhanced T1-weighted MR, the lesions are typically slightly hypointense to normal glandular tissue and clearly hypointense to fat. Contrast-enhanced MR may make the more vascular portions of the matrix isointense to surrounding glandular tissue or fat. The appearance on T2-weighted images is quite diverse. The glandular volume of the tumor tends to be bright on heavily T2-weighted images due to the water content of glandular spaces and/or mucoid stroma (Figs. 22.1, 22.2, 22.4, and 22.9). The relative amount of this in any particular lesion is not predictable (Figs. 22.4G vs. 22.4H). Densely cellular areas will behave the same as any solid, cellular neoplasm. Areas of fibrosis or chondroid stroma or calcification and ossification will produce foci of low signal intensity on T2-weighted images.
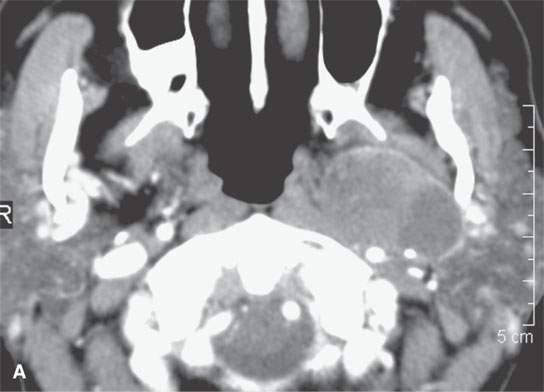
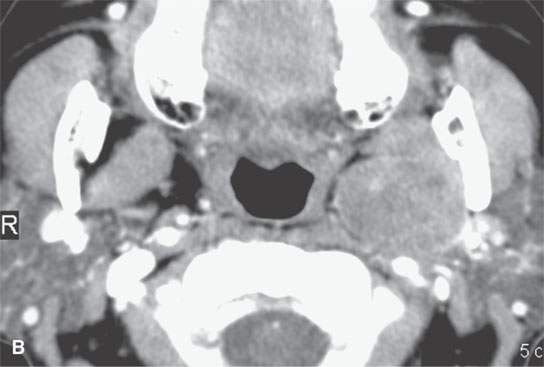
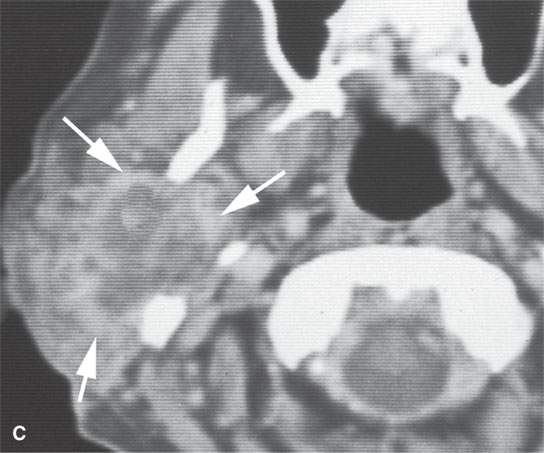
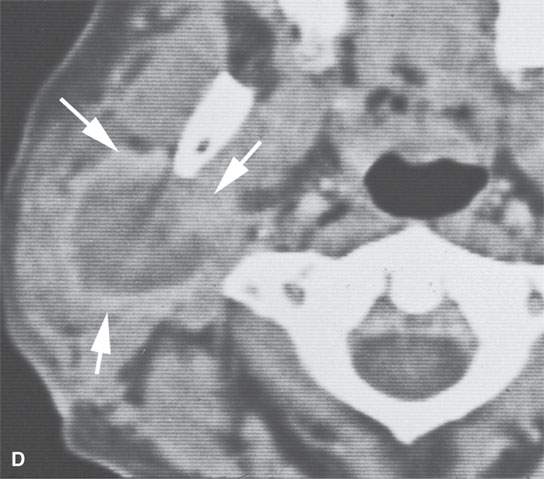
FIGURE 22.10. Contrast-enhanced computed tomography of a well-circumscribed parapharyngeal monomorphic adenoma (A, B) and a monomorphic adenoma (C, D) with more aggressive margins (arrows). Both were benign.
The clinical course of mixed tumors is almost entirely related to the adequacy of treatment. These tumors must be excised with a margin of surrounding normal tissue. If the capsule is violated during surgery, the tumor will likely spill into the operative site (Fig. 22.9). Fine needle biopsy does not seem to raise the risk of spread. Incisional biopsy or subtotal removal is a mistake and can be avoided by preoperative imaging that allows one to know the full extent of the lesion before it is approached surgically. Biopsy across a mucosal surface should be avoided, as it might subsequently require resection and repair of a violated mucosal area. Recurrences are best diagnosed by MR. The usually bright foci of recurrence are readily distinguished from postoperative scar on heavily T2-weighted, short T1 inversion recovery (STIR) images or contrast-enhanced fat-suppressed images (Fig. 22.9). Radiotherapy is occasionally used to slow the growth of recurrent disease when reoperation is not desirable or possible.
Carcinoma arising in a benign mixed tumor or carcinoma ex pleomorphica is an uncommon occurrence. Often, this pathologic diagnosis is a surprise clinically; however, if an unusually aggressive feature is present, such as facial nerve palsy, or if nodal spread or local invasion is present, one should suspect this diagnosis even if the initial histologic review suggests a benign mixed tumor (Fig. 22.11).
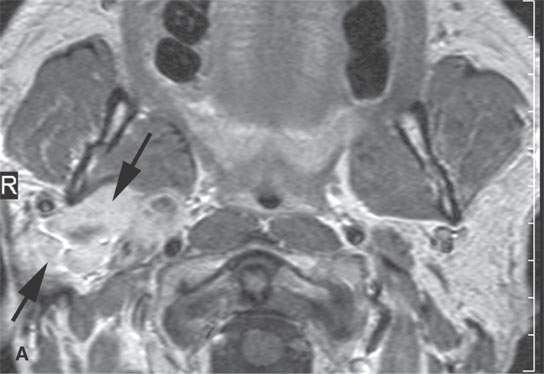
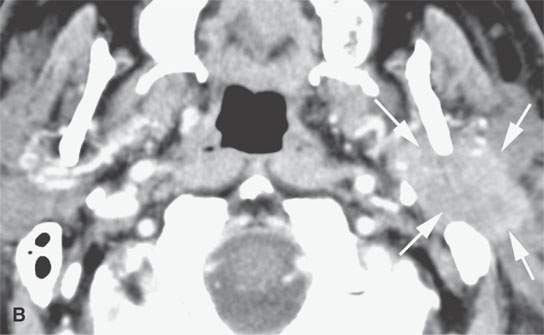
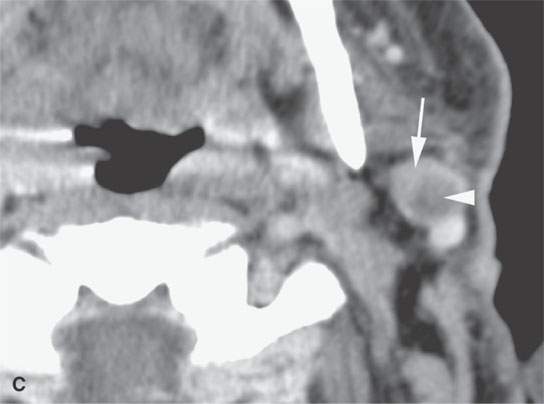
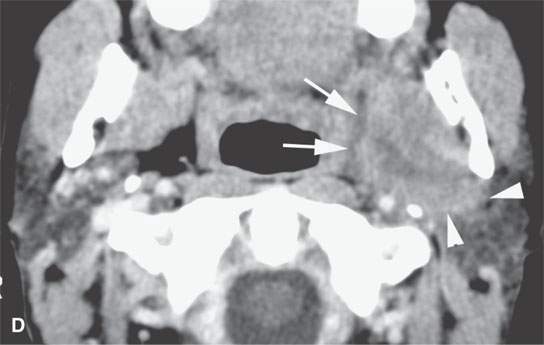
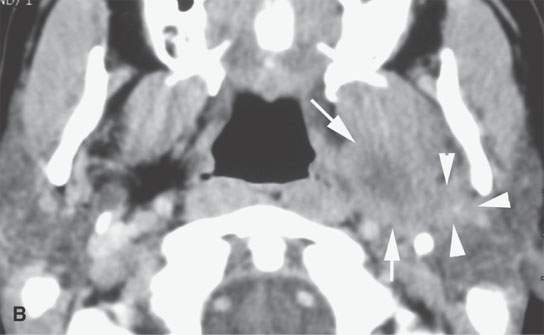
FIGURE 22.11. Three patients with carcinoma arising from a benign mixed tumor (carcinoma ex pleomorphica). A: Contrast-enhanced T1-weighted images showing the multifocal cancer with aggressive margins in the deep portion of the parotid gland and adjacent parapharyngeal space. B, C: Contrast-enhanced computed tomography (CECT) showing the parotid-origin cancer (C) and related node (arrow in D) containing a metastatic deposit. D, E: CECT of a cancer arising from a parapharyngeal BMT; the parapharyngeal fat is infiltrated (arrows), and the lateral margin of the mass suggests locally aggressive behavior (arrowheads) (D); the otherwise well-circumscribed mass (arrows) invades laterally along the pterygoid plexus (arrowheads) (E).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree