Benign Focal Prostate Lesions
Etiology
Benign focal lesions of the prostate include benign prostatic hyperplasia (BPH) (see Chapter 72 ), congenital cysts, acquired cysts, prostatitis (acute bacterial, chronic bacterial, chronic pelvic pain syndrome [inflammatory and noninflammatory], and asymptomatic prostatitis), prostatic abscess, and prostatic calcification.
The National Institutes of Health classification of prostatitis syndromes provides a useful conceptual framework. Categories I and II reflect acute and chronic bacterial prostatitis, respectively. Category III, known as chronic prostatitis/chronic pelvic pain syndrome, constitutes the vast majority (>90%) of cases and is divided into IIIA (inflammatory) and IIIB (noninflammatory). Category IV refers to asymptomatic inflammatory prostatitis, usually diagnosed incidentally.
Prevalence and Epidemiology
Prostatitis is perhaps the most common urologic complaint in men younger than 50 years of age and affects 11% to 16% of American men over the course of their lifetime.
Clinical Presentation
The presentation of benign prostate disease varies according to the particular pathologic process. For example, acquired prostate cysts and calcification are typically asymptomatic, whereas prostatitis ranges from incidentally detected asymptomatic conditions to symptomatic cases.
Pathology
Any part of the prostate gland can be involved by prostatitis, abscess, or calcification. Acquired cysts are located in a paramedian distribution. In prostatitis, there is an increased number of inflammatory cells. Cysts and calcifications are benign processes.
Imaging
The most commonly used diagnostic imaging techniques for prostate evaluation are transrectal ultrasound (TRUS) and MRI. Benign findings such as cysts and calcifications are typically incidental, usually found on routine investigation for other conditions; most benign processes such as BPH and prostatitis require little investigation. TRUS can provide high-resolution images of the prostate and real-time guidance for intervention such as biopsy, aspiration, and drainage, without the use of radiation. Magnetic resonance imaging (MRI) accurately delineates the internal prostatic anatomy but is not routinely used for the investigation of benign prostate lesions owing to its high cost and relatively limited availability. Relative to these modalities, radiography and computed tomography (CT) have limited roles in the evaluation of most prostate processes.
Treatment
Antibiotics are the mainstay of treatment for prostatitis. Other treatments, including both pharmacologic and nonpharmacologic approaches, have been assessed as potential treatments for chronic prostatitis and pelvic pain syndromes. Prostatic abscess drainage is the only indication for surgical intervention in benign prostatic disease.
Specific Lesions
Acute Bacterial Prostatitis
Etiology.
Acute bacterial prostatitis is most commonly caused by aerobic gram-negative rods, in particular Escherichia coli and Pseudomonas species. Bacteria may ascend to the prostate by reflux of infected urine into the prostatic duct, by lymphatic or hematogenous dissemination, or during interventions such as prostatic biopsy. Emphysematous prostatitis occurs secondary to infection with gas-forming organisms; while rare, it is associated with high mortality.
Prevalence and Epidemiology.
Acute bacterial prostatitis is rare and is seen in less than 5% of patients with prostatitis.
Clinical Presentation.
Acute bacterial prostatitis usually manifests as an acute illness with fever, chills, lower back and perineal pain, urinary frequency and urgency, and dysuria. Rectal palpation usually reveals an enlarged, exquisitely tender prostate gland. The diagnosis of acute bacterial prostatitis is based primarily on clinical findings, in association with positive results of urinalysis and urine culture.
Pathology.
The prostate may be focally or diffusely involved. In acute infection, the prostate enlarges secondary to infection and inflammation. An increased number of inflammatory cells is seen in prostate biopsy specimens.
Imaging.
Radiologic examinations usually are not required, unless severe infection and/or abscess is suspected. When indicated, ultrasonography and MRI are favored for their high soft tissue contrast, multiplanar capabilities, and lack of ionizing radiation. However, imaging modalities may be limited in the differentiation of prostatitis from BPH and prostate cancer. Prostatic tenderness associated with acute prostatitis may preclude TRUS.
Computed Tomography.
In acute prostatitis, the gland may appear normal or focally or diffusely enlarged. There is homogeneous attenuation with possible nonspecific stranding in the periprostatic fat ( Figure 73-1 ).

Magnetic Resonance Imaging.
The prostate may appear normal on MRI in the setting of acute prostatitis. It may be focally or diffusely enlarged. Single or multiple foci of high signal intensity on T2-weighted images may be seen ( Figure 73-2 ). T1-weighted imaging is nonspecific owing to limited delineation of the internal structure of the prostate. On postcontrast T1-weighted images, the areas of inflammation enhance with gadolinium. Diffusion weighted imaging (DWI) has been reported to yield higher apparent diffusion coefficients (ADCs) in prostatitis cases than in malignancy, but with significant overlap; caution must be taken to not mistake malignancy for acute or chronic prostatitis.

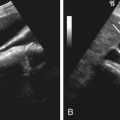
Ultrasound.
By TRUS, the prostate gland may be of normal or enlarged size and may appear normal or demonstrate focal or diffuse areas of mixed echogenicity. Doppler vascularity may be increased. Other ultrasound features of prostatitis include dilatation of the periprostatic venous plexus, elongated seminal vesicles, and thickening of the inner septa. These features can resemble both the changes of BPH and prostatic carcinoma.
Imaging Algorithm.
Radiologic imaging is rarely required and only in the instance when severe infection and/or abscess is suspected.
Differential Diagnosis.
The diagnosis of acute bacterial prostatitis is based primarily on clinical findings, in association with positive results on urinalysis and urine culture. Prostatitis cannot be definitively differentiated from prostate cancer by imaging alone. Further confounding this point, in the presence of acute infection, the prostate-specific antigen (PSA) value may be elevated. Investigation for prostate cancer should be initiated if the PSA level fails to return to normal levels after therapy.
Treatment.
Antibiotics are the mainstay of treatment for acute bacterial prostatitis. Full response and resolution are expected. Radiologic or surgical interventions are usually not required for acute prostatitis unless complicated by abscess formation.
- •
The diagnosis of acute bacterial prostatitis is based primarily on clinical and laboratory findings.
- •
Antibiotics are the mainstay of treatment.
Prostatic Abscess
Etiology.
Prostatic abscess can occur from local spread of infection, hematogeneous seeding, or instrumentation of the prostate or lower urinary tract or may be secondary to preexisting prostatitis. E. coli and Staphylococcus are the most commonly involved organisms. Early antibiotic therapy has reduced the incidence of abscess as a complication of prostatitis.
Prevalence and Epidemiology.
Prostate abscess is rare, diagnosed only in 0.2% of patients with urologic symptoms and in 0.5% to 2.5% of patients hospitalized for prostatic symptoms.
Clinical Presentation.
A high degree of clinical suspicion and close monitoring of response to treatment is required to make the diagnosis, as the symptoms of prostatic abscess are similar to those of acute prostatitis and other lower urinary tract inflammatory conditions. Prostatic abscess should be suspected when there is failure to respond to treatment of acute bacterial prostatitis.
Pathophysiology.
Prostatic abscess may involve any part of the gland. If it occurs at the apex, spontaneous bladder or proximal prostatic urethra fistula formation may occur. If the abscess is situated at the base of the gland, it may extend through perirectal tissues into the ischiorectal fossa, resulting in rectal and perineal fistulas.
Pathology.
Inflammatory cells and bacteria are seen in abscess aspirates.
Imaging.
The imaging features of prostate abscess are similar to those of abscess in other areas of the body. They range from focal tissue abnormality to gas-containing fluid collections.
Computed Tomography.
CT allows for rapid, comprehensive evaluation of prostatic abscess and assessment for involvement of periprostatic tissue, organs, and vascular structures.
CT features of prostatic abscess include focal or diffuse enlargement, heterogeneous attenuation, and low-density collection ( Figure 73-3 ). Prostatic abscess may be unilocular or multilocular, may contain gas, and enhances peripherally after administration of intravenous contrast. Periprostatic fat and adjacent seminal vesicles and bladder may be secondarily infected.

Magnetic Resonance Imaging.
MRI features closely parallel those found on CT, with the added benefit of superior soft tissue contrast (though with longer examination time). A prostatic abscess demonstrates well-defined high signal intensity on T2-weighted images but is usually not well seen on T1-weighted images without contrast enhancement. On administration of intravenous gadolinium, it shows peripheral enhancement of variable intensity. Spread of infection or complications of chronic disease such as fistula formation may be evident. In patients with prostatic abscess, T2-weighted MRI shows a fluid-containing lesion with radiating, streaky areas of low signal intensity.
Ultrasound.
On ultrasound evaluation, prostatic abscess appears as a heterogeneous mass that may contain internal echoes, septations, and shadowing. When there is marked edema, a hypoechoic halo may be observed on gray-scale ultrasonography. When air is present, shadowing may limit full visualization of the abscess and gland. There may be increased Doppler vascularity secondary to hyperemia and inflammation.
TRUS-guided drainage may be useful in the treatment of prostate abscess. Abscesses greater than 1.5 cm are usually aspirated; aspiration of the infected fluid in combination with intravenous antibiotics has a success rate of over 80% in curing prostate abscesses. Close follow-up is key to prevent chronic prostatitis.
Imaging Algorithm.
Cross-sectional imaging is recommended when there is a clinical suspicion of prostate abscess, usually owing to failure of prostatitis to respond to appropriate treatment ( Figure 73-4 ). Ultrasound and MRI are preferred to CT because of superior soft tissue contrast resolution. Ultrasound can guide transrectal aspiration. CT also demonstrates prostate abscess well and can guide transperineal drainage.

Differential Diagnosis.
Acute bacterial prostatitis has a similar presentation. A high index of suspicion is required to diagnose prostatic abscess. The presence of the abscess is confirmed with ultrasound, MRI, or CT.
Treatment.
Medical treatment with broad-spectrum antibiotics alone is usually unsuccessful. The presence of a prostatic abscess is an indication for drainage.
- •
A high index of suspicion is required for diagnosis.
- •
Adequate treatment is required to prevent sepsis and long-term complications such as formation of a pelvic fistula.
- •
Image-guided or surgical drainage with broad-spectrum antibiotics is the treatment of choice.
Chronic Bacterial Prostatitis
Etiology.
The same organisms that produce acute prostatitis also have been implicated in chronic prostatitis. Chronic prostatitis may follow acute prostatitis, but some clinicians believe that noninfective venous congestion of the prostate may be the initial change that predisposes to subsequent chronic infection. Separately, granulomatous prostatitis has been reported as a rare form of chronic inflammation. A diagnosis established only by biopsy, granulomatous prostatitis can be seen in infectious (including Mycobacterium ), postsurgical or postradiation, and idiopathic settings.
Prevalence and Epidemiology.
Chronic prostatitis is rare, occurring in 5% to 10% of all men with prostatitis.
Clinical Presentation.
Chronic bacterial prostatitis manifests as chronic pain and recurrent urinary tract infections.
Pathology.
Any part of the prostate may be involved. There are an increased number of inflammatory cells in the parenchyma.
Imaging.
A chronically inflamed gland is usually small, but it may be of normal size or enlarged if BPH is present concurrently. Imaging cannot confidently differentiate prostatitis from BPH and prostate cancer.
Computed Tomography.
CT has not been widely used in the investigation of chronic prostatitis. The prostate may be small, hypoattenuating, and may contain calcifications.
Magnetic Resonance Imaging.
Chronic prostatitis often demonstrates diffuse streaky areas of low signal intensity on T2-weighted images, known as the “watermelon” sign. T1-weighted imaging is nonspecific, and the affected prostate may not enhance after administration of gadolinium. When chronic infection involves the peripheral zone, its appearance is difficult to distinguish from that of prostate cancer; biopsy is required for definitive diagnosis.
Ultrasound.
Similar to findings on CT and MRI, chronic prostatitis can be focal or diffuse and mostly appears as an irregular, hypoechoic area in the peripheral zone on ultrasound evaluation.
Imaging Algorithm.
See the imaging algorithm in Figure 73-4 .
Differential Diagnosis.
Chronic bacterial prostatitis has a similar presentation to that of chronic pelvic pain. Chronic prostatitis cannot be definitively distinguished from prostate cancer by imaging alone and may require prostate biopsy.
Treatment.
Both pharmacologic and nonpharmacologic therapies have been evaluated in the treatment of chronic prostatitis. Surgery usually is not required.
- •
Antibiotics are the mainstay of treatment of chronic prostatitis.
Prostate Cysts
Etiology.
Prostate cysts may be congenital or acquired. Acquired cysts include retention cysts, ejaculatory duct cysts, and cystic degeneration of BPH. Cystic carcinoma of the prostate is rare.
Cystic degeneration of BPH is the most common cause of cystic lesions in the prostate. They are located in the transitional zone and are seen as small cysts within the nodules of BPH.
Retention cysts are 1- to 2-cm, smooth, thin-walled unilocular cysts that occur in the fifth to sixth decades. They occur as a result of acquired obstruction and dilation of glandular acini and may be found in all zones of the prostate.
Ejaculatory duct cysts are typically small and are located in the lateral aspects of the prostate gland. They may accompany ejaculatory duct obstruction/obliteration with azoospermia.
Prevalence and Epidemiology.
The exact prevalence of prostate cysts is unknown. However, cystic degeneration of BPH is common.
Clinical Presentation.
Most patients are asymptomatic, and these cysts are detected incidentally. Rarely, they become symptomatic when inflamed or infected or when they are large, causing urinary outflow obstruction or infertility secondary to ejaculatory duct obstruction.
Pathophysiology.
Midline cysts are usually congenital because of anomalies of the müllerian duct system. Acquired cysts are paramedian in location and most commonly associated with BPH.
Pathology.
Prostate cysts are benign.
Imaging.
Prostate cysts are usually asymptomatic and found incidentally.
Computed Tomography.
Prostate cysts are low-density lesions in the prostate. MRI and ultrasound are superior to CT in delineating prostate cysts.
Magnetic Resonance Imaging.
Cysts are generally uniformly high in signal intensity on T2-weighted images secondary to their fluid content. These cysts demonstrate variable signal intensity on T1-weighted images depending on the presence of infection or hemorrhage.
Ultrasound.
Acquired prostate cysts are anechoic lesions that occur most commonly in the transitional zone as a result of degeneration of BPH. They also may be seen in the peripheral zone.
Imaging Algorithm.
These cysts are usually discovered incidentally. No further imaging is required.
Treatment.
Transurethral resection or aspiration should be considered the first line of management of symptomatic cysts. Open resection may also be required.
- •
Acquired cysts are usually incidental findings. Unless symptomatic, no treatment is required.
Prostate Calcification
Etiology.
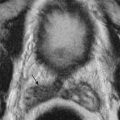
Primary, or idiopathic, prostatic calcification develops in the acini of the prostatic parenchyma. The cause is unknown, and the relationship to infection is also unclear. Some believe that primary prostatic calcification develops by calcification of the corpora amylacea, forming “prostatic calculi” ( Figure 73-5 ). These are small, round or ovoid bodies seen in the lumen of the prostatic acini that may be derived from desquamated epithelial cells and proteinaceous material.

Secondary prostatic calcification may be seen in association with BPH or carcinoma, infection, radiation therapy, and diabetes. Calculi also may develop in an abscess cavity or diverticulum.
Prevalence and Epidemiology.
A common finding, prostate calcification increases with age, most prominently between the ages of 40 and 70 years.
Clinical Presentation.
Most cases are asymptomatic. However, prostate calculi may cause obstruction, pain, infection, and hematuria.
Pathology.
Calcification can be found in any part of the gland.
Imaging.
Prostate calcifications are larger than prostate calculi. Calculi occur in the lumen of prostate acini. Calcification occurs in the parenchyma and may be focal or diffuse, involve a small or large area, and occur periurethrally or at the surgical capsule.
Computed Tomography.
CT demonstrates high-density calcification within the prostate gland.
Magnetic Resonance Imaging.
MRI is less sensitive than CT and ultrasound in the detection of prostate calcification. Calcifications are seen as areas of low signal intensity.
Ultrasound.
Prostatic calcification has typical features on ultrasonography: it is echogenic, and associated acoustic shadowing may obscure visualization of the remainder of the gland.
Differential Diagnosis.
Prostatic calcification is almost always asymptomatic. No clinical or laboratory data can determine its cause. The PSA level and fasting glucose value should be checked if the possibility of prostate cancer or diabetes is suspected.
Treatment.
No medical treatment is required when prostate calcification is asymptomatic. Symptoms may occur in the setting of superimposed infection in which antibiotics are the mainstay of treatment. When obstructive or chronic infective symptoms occur, surgical treatment may be needed.
In those who are symptomatic, calculi may be removed transurethrally. Rarely, surgical prostatectomy may be indicated in patients with intractable infection.
- •
Prostate calcification is usually asymptomatic.
- •
Most benign prostate processes do not require radiologic investigation unless atypical signs or symptoms are present.
- •
Antibiotics are the mainstay of treatment for acute and chronic prostatitis. Drainage is usually indicated for prostatic abscess.
- •
Imaging tests alone cannot definitively differentiate prostatitis and prostate carcinoma.
Malignant Focal Prostate Lesions
Prostate cancer is a common disease and an important health issue for men worldwide. The diagnosis and management of prostate cancer is highly complex, stemming from the uncertain natural history of the disease and its unpredictable biologic behavior. There is a high prevalence of the disease; autopsy series have revealed small prostate cancers in as many as 29% of men between ages 30 and 40 and 64% of men between ages 60 and 70. However, a high proportion of prostate cancer fails to develop into clinically significant symptomatic cancer. There is no perfect method to determine which patients will have disease that will progress. Factors such as a high PSA level, Gleason score, and stage are all useful for predicting outcome, but algorithms that combine stage, grade, and PSA level to predict pathologic stage or prognosis perform better than these individual factors alone. Imaging plays an important contributory role in the management of prostate cancer.
Etiology
The cause of prostate cancer is unknown. There are a number of risk factors, including increasing age (prostate cancer is rarely seen in men younger than age 40), ethnicity (African Americans are at greatest risk), diet, consumption of antioxidants, and a family history of prostate cancer. Recent genetic mapping studies have identified RNASEL and MSR1 as potential prostate cancer susceptibility genes.
Prevalence and Epidemiology
According to the National Cancer Institute Surveillance, Epidemiology, and End Results Program (seer.cancer.gov), a total of 220,800 new cases of prostate cancer were estimated in 2015, accounting for 13.3% of all new cancer cases. There were 27,540 estimated deaths from prostate cancer in 2015, resulting in 4.7% of all cancer deaths. Approximately 14% of men will develop prostate cancer at some point during their life.
Prostate cancer incidence increased dramatically in the early 1990s owing to earlier diagnosis with the introduction of PSA blood testing. Prostate cancer incidence continues to increase, although at a slower rate. This may be attributable to increased screening through PSA testing. Although prostate cancer mortality rates have declined over the past decade, there is no evidence to link PSA screening to this decrease in mortality.
Clinical Presentation
Prostate carcinoma is often asymptomatic. Symptomatic disease may manifest as prostatism and occasionally hematuria. Other manifesting symptoms may include bone pain and/or pathologic fractures related to bone metastases, uremia secondary to distal ureteral infiltration, and local hemorrhage resulting from tumor necrosis or obstruction. Digital rectal examination (DRE) may be normal or demonstrate an irregular, enlarged gland. Prostate cancer is characterized clinically by the serum PSA level; tumor, node, metastasis (TNM) stage; and Gleason score.
Prostate-Specific Antigen
The PSA level is a strong indicator of stage and prognosis and is helpful in monitoring response to therapy. PSA serum levels must be interpreted carefully with regard to patient age, gland size, recent DRE or biopsy, and the presence of infection, all of which can increase the PSA level. Currently, a PSA serum threshold of 4.0 ng/mL is widely used as the threshold above which further investigations are performed for prostate carcinoma. The probability of detecting prostate cancer increases as the PSA rises. Between 0 and 2 ng/mL, 1% of patients have prostate cancer; between 2 and 4 ng/mL, 15% have prostate cancer; between 4 and 10 ng/mL, 25% have prostate cancer; and for a PSA level greater than 10 ng/mL, more than 50% will have prostate cancer. Therefore, some have recommended the use of lower threshold values (<4 ng/mL) to avoid missing prostate cancers and to increase the likelihood that prostate cancers are detected at a curable stage. Although this may lead to detection of more cancers, it also may result in overdiagnosis of cancers (especially in older men) that may not manifest clinically during the patient’s lifetime.
The PSA density is obtained by dividing the PSA by the prostate size. This helps distinguish those with an abnormally high PSA from those with an elevated PSA secondary to BPH. A PSA density of 0.15 or greater has been proposed as a cutoff level for recommending prostate biopsy in men with serum PSA levels between 4 and 10 ng/mL and no suspicion of prostate cancer on DRE or transrectal ultrasonography (TRUS). However, the correlation between PSA density and the presence of prostate cancer is not absolute.
PSA velocity is the rate of increase of the PSA level. A PSA velocity increase of greater than 0.75 ng/mL per year indicates a significant risk for prostate cancer regardless of the absolute serum PSA value.
Pathology
Prostate cancer most commonly occurs in the peripheral zone (70% of cancers), followed by the transitional zone (20%) and central zone (5%).
Ninety-five percent of prostate cancers are adenocarcinomas. Approximately 4% have transitional cell morphology and are thought to arise from the urothelial lining of the prostatic urethra. More rarely, a squamous cell type is found and, very rarely, a sarcoma (0.1% to 0.2%). A few cases have a neuroendocrine morphology believed to arise from neuroendocrine stem cells normally present in the prostate. Prostate cancer is graded histologically on a scale of 1 to 4 according to the cell differentiation and degree of anaplasia, as follows:
- •
GX: Grade cannot be assessed
- •
G1: Well differentiated (slight anaplasia) (Gleason 2 to 4)
- •
G2: Moderately differentiated (moderate anaplasia) (Gleason 5 to 6)
- •
G3 to 4: Poorly differentiated or undifferentiated (marked anaplasia) (Gleason 7 to 10)
Two thirds of prostate cancers have a mix of tumor grades. To determine the prognosis and aggressiveness of a tumor, a Gleason score is assigned histologically. Pathologists identify the two most common patterns of cells in the tissue and assign a Gleason grade to each on a scale of 1 to 5. The two grades represent the dominant and minor grade in the specimen and combine to make up the Gleason score. The higher the Gleason score, the more likely it is the cancer will grow and spread rapidly and the worse the prognosis, as follows:
- •
Gleason score 2 to 4: Well differentiated; minimal risk for death from prostate cancer in the following 15 years (indicates ~95% chance for surviving 15 years without aggressive treatment)
- •
Gleason score 5 to 6: Moderately well differentiated; modest risk for death from prostate cancer that increases slowly over at least 15 years of follow-up
- •
Gleason score 7 to 10: Moderately to poorly differentiated, with a 15-year survival rate of 15% to 40% even when cancer is diagnosed as late as 74 years of age
Atypical cells and prostatic intraepithelial neoplasia (PIN) diagnoses are made when a prostate biopsy specimen does not look frankly neoplastic on histologic examination but the cells are abnormal. PIN can be further divided into low and high grades. The significance of low-grade PIN in relation to prostate cancer remains unclear, but the presence of atypical cells or high-grade PIN increases the likelihood of the presence of prostate cancer in the gland. There is a 30% to 50% likelihood of finding prostate cancer in a later biopsy specimen when high-grade PIN is initially discovered. For this reason, repeat biopsies are generally recommended.
Imaging
Computed Tomography
CT has a limited role in assessing prostate cancer because it is usually unable to depict early-stage (T1 and T2) tumors ( Figure 73-6 ). CT may demonstrate locally advanced disease with extracapsular extension, seminal vesicle involvement, and invasion into the mesorectum, rectum, bladder, and levator ani ( Figure 73-7 ). Pelvic and abdominal lymph nodes also may be demonstrated. Evidence-based guidelines recommend the use of CT for distant prostate cancer staging in patients with a PSA greater than 20 ng/mL, Gleason score greater than 7, and/or clinical tumor stage T3 or higher.


Magnetic Resonance Imaging
The initial role of prostate MRI was for locoregional staging in patients with biopsy-proved cancer ( Figure 73-8 ). T1- and T2-weighted images provided anatomic information to help distinguish T2 and T3 disease (i.e., identify extracapsular extension) and evaluate for nodal disease ( Boxes 73-1 and 73-2 , Figures 73-9 to 73-13 ). Rapid growth of MRI technology and reader experience over the past 2 decades has led to a greatly expanded role for prostate MRI. The introduction of so-called multiparametric MRI has expanded the role of MRI in prostate cancer imaging to include tumor detection, localization, characterization, surveillance, and guidance for targeted biopsy. The accuracy of prostate MRI in local staging has improved with time, most likely owing to improvements in MRI technology, better understanding of morphologic criteria used to diagnose extracapsular extension or seminal vesicle invasion, and increased reader experience.

- •
An irregular, spiculated, or angulated prostate margin
- •
Capsular retraction
- •
Asymmetry of the neurovascular bundle
- •
Tumor envelopment of the neurovascular bundle
- •
Obliteration of the rectoprostatic angle
- •
Tumor bulge into the periprostatic fat
- •
Broad tumor contact with the surface of the capsule
- •
Extracapsular tumor
- •
Disruption or loss of the normal architecture of the seminal vesicle
- •
Focal low signal intensity in the seminal vesicle
- •
Enlarged low signal intensity ejaculatory ducts
- •
Enlarged low signal intensity seminal vesicle
- •
Obliteration of the acute angle between the prostate and the seminal vesicle (best seen on sagittal images)
- •
Demonstration of direct tumor extension from the base of the prostate into and around the seminal vesicle





There is currently no consensus regarding optimal patient preparation for prostate MRI. Most practices suggest the use of an enema before the examination, with evacuation immediately preceding the MRI to diminish the amount of stool and air in the rectum, which cause susceptibility artifact (particularly on diffusion weighted sequences). Some recommend abstinence from ejaculation for 3 days before prostate MRI to maintain seminal vesicle distention. An antispasmodic agent (e.g., glucagon) can be used to minimize bowel peristalsis, although it introduces increased cost and potential for adverse drug reactions. In the ideal scenario, it is universally recommended that the MRI is scheduled at least 6 weeks or more after TRUS biopsy to allow for resolution of postprocedural hemorrhage and inflammation.
Protocol Considerations.
Protocols for optimal multiparametric evaluation of the prostate continue to evolve. The key is to obtain consistent image quality with an adequate signal-to-noise ratio (SNR) to allow for confident interpretation. At 1.5 T, most experienced readers think that insertion of an endorectal coil in addition to the use of a standard pelvic phased array radiofrequency coil is necessary to obtain adequate SNR in the prostate. Endorectal coil placement at 3 T produces even higher SNR, with improved image quality, higher spatial resolution, and significantly improved localization and staging performance for both experienced and less experienced radiologists. However, the endorectal coil also can be associated with deformation of the prostate, increased cost and examination time, artifacts (specifically susceptibility), and patient discomfort (which may lead to reluctance to undergo prostate MRI). Some institutions now image exclusively at 3 T without the use of an endorectal coil. Individual centers should tailor their protocols to achieve optimal image quality as they deem appropriate.
Multiparametric Magnetic Resonance Imaging.
The combination of anatomic and functional evaluation of the prostate constitutes the elements of multiparametric MRI (mpMRI). Integration of T2-weighting imaging, diffusion weighted imaging, and perfusion imaging (through dynamic contrast-enhanced acquisitions) has led to a rapid growth in the understanding of the morphology, composition, and enhancement characteristics of prostate cancer and its mimics.
T2-Weighted Imaging.
T2-weighted imaging is the workhorse of mpMRI because it demonstrates the zonal anatomy of the prostate while allowing identification and characterization of focal lesions. Multiplanar fast spin echo T2-weighted images of the prostate are typically obtained in small field-of-view (FOV) pulse sequences in axial, coronal, and sagittal planes. The axial and coronal sequences should be obtained in a plane oblique to the axis of the prostate to preserve the normal zonal architecture and prevent volume averaging. Large FOV axial (and possibly coronal) T2-weighted sequences are also obtained to the level of the aortic bifurcation to evaluate for nodal disease. T2-weighted sequences are also useful in detecting extracapsular extension and seminal vesicle invasion ( Tables 73-1 and 73-2 ).