Chapter Outline
Splenunculi, Splenic Ectopia, and Splenosis
Splenic Infection and Inflammatory Disease
Fungal Abscess and Microabscess
Mycobacterium avium Complex Infection
Pneumocystis jiroveci Pneumonia
Lymphoma or Metastatic Disease
Although it is affected by many diseases, the spleen is not considered a great challenge to the radiologist. In daily practice, the size and shape of the spleen typically are subjectively evaluated, and if there is no obvious splenomegaly or focal abnormality, the spleen usually is ignored. However, when abnormalities are detected, the radiologist plays an important role in providing a diagnosis and directing further clinical management.
Each imaging modality, including ultrasound, computed tomography (CT), magnetic resonance imaging (MRI), and scintigraphy, has specific advantages and limitations in assessing the spleen. Plain radiographs of the abdomen can detect splenomegaly ( Fig. 105-1 ) and splenic calcifications ( Box 105-1 ) but otherwise do not have a significant diagnostic role. Currently, angiography is used only for therapeutic purposes, such as splenic embolization in trauma, hypersplenism, or rare vascular tumors. 18 F-fluorodeoxyglucose positron emission tomography (FDG PET) combined with CT (FDG PET/CT) is becoming increasingly useful in the evaluation of malignant disease and has a high negative predictive value in predicting a malignant neoplasm in patients with solid-appearing splenic masses. Because of a perceived high risk of bleeding, percutaneous biopsies of the spleen are not often performed in general practice. They are, however, relatively safe.

Cysts
Hemangioma
Hamartoma
Lymphangioma
Splenic artery
Granulomas
Tuberculosis
Histoplasmosis
Pneumocystis jiroveci pneumonia
Hydatid disease
Hematomas
Hemosiderosis
Infarcts
Metastases
Sarcoidosis
Sickle cell disease
Gamna-Gandy bodies
Thorotrast (not true calcifications)
The various imaging appearances and characteristics of benign and malignant splenic lesions are described in this chapter. Recognizing the range of the imaging and pathologic features of splenic abnormalities will narrow the differential diagnosis and help in planning further management.
A unique feature of the spleen on multidetector CT and MRI is the inhomogeneous enhancement pattern during the first minute after the intravenous administration of contrast material, which is most pronounced in the arterial phase (first 25-35 seconds). The enhancement pattern varies with different injection rates and tends to be more prominent in the presence of splenomegaly. These patterns have been described as serpentine, arciform, striped (i.e., zebra spleen), mottled, focal, and diffuse heterogeneity. This normal phenomenon is probably the result of differential flow through the vascular sinuses and cords of the red and white pulp. In most patients, this heterogeneity resolves after 60 to 70 seconds and should not be confused with disease. Keeping this phenomenon in mind, the normal spleen should be homogeneous on all imaging modalities.
True lesions involving the spleen can be categorized as diseases manifesting with focal lesions, multifocal lesions, or a diffuse infiltrating pattern ( Box 105-2 ). Although often asymptomatic, focal abnormalities must raise the suspicion of a pathologic condition. Rarely, when the lesions are large, they may cause symptoms.
Solitary Lesions
Solitary focus of any typically multifocal disease
Benign neoplasms: hemangioma, lymphangioma, hamartoma, inflammatory pseudotumor, peliosis, littoral cell angioma, sclerosing angiomatoid nodular transformation
Solitary pyogenic abscess
Multiple Focal Abnormalities
Trauma: lacerations, fractures, intrasplenic and subcapsular hematomas
Splenic rupture
Abscess: bacterial, fungal, granulomatous
Calcified granulomas
Sarcoidosis
Lymphangiomatosis, hemangiomatosis, peliosis
Lymphoma, lymphoproliferative disorders
Gaucher’s disease
Metastatic disease
Hemangioma, hemangioendothelioma, peliosis
Diffuse Disease Without Focal Lesions
Congestive disease: portal hypertension, splenic vein occlusion, or congestive heart failure
Inflammatory disease: various infections (e.g., tuberculosis) and inflammation (e.g., sarcoid)
Hyperplastic splenomegaly: hypertrophy resulting from removal of abnormal blood cells from the circulation (e.g., polycythemia vera)
Infiltrative (e.g., Gaucher’s disease, hemosiderosis, malignant disease)
If the patient has a known disease, such as metastases, lymphoma, or tuberculosis, a general assumption is made that the focal abnormality represents splenic involvement. Ultimately, it is often the radiologist who has to decide whether further investigations are indicated or follow-up is more appropriate.
Splenunculi, Splenic Ectopia, and Splenosis
Splenunculi, also known as accessory spleens, splenules, and supernumerary spleens, are a common finding. They are found in approximately 10% to 30% of autopsies and in 16% of patients undergoing abdominal CT. The normal spleen is formed after multifocal development of splenic foci and subsequent fusion. Splenunculi are formed when one or more of these splenic foci fail to fuse.
Accessory spleens are a few millimeters to several centimeters in the greatest diameter. They are typically supplied by branches of the splenic artery and demonstrate the same imaging and enhancement characteristics as normal splenic tissue. They are most commonly located medial to the spleen in or near its hilum (75%) and adjacent to the tail of the pancreas. Less common locations are within the pancreatic tail (17%), the gastrosplenic or splenorenal ligaments, the wall of the stomach or bowel, the mesentery or omentum, and the pelvis and scrotum ( Fig. 105-2 ). Those found in the pancreatic tail are usually small and rarely noticed radiologically; if detected, they are often misdiagnosed as hypervascular tumors.

In patients with heterotaxia, left isomerism, or bilateral left-sidedness, many bilateral spleens or splenunculi may be present. Splenosis is autotransplantation of splenic tissue after disruption of the splenic capsule by trauma or surgery. Splenosis has been described in up to 74% of patients who underwent splenectomy after trauma, and it is often detected years after the initial trauma. These splenic implants closely resemble splenunculi on histologic examination, and distinction between these two conditions usually is not possible. Compared with accessory spleens, these implants are more numerous and variable in size and shape, and they are supplied by small perforating vessels arising at the site of implantation. They are typically located in the abdomen but may be found in various locations, including the pelvis, thorax, and scars.
Splenunculi and splenosis usually have little clinical significance, although there are exceptions. When splenectomy is performed to control hematologic disorders such as idiopathic thrombocytopenic purpura or lymphoma, hypertrophy of an accessory spleen may cause or harbor the site of recurrent disease. Accessory spleens also may mimic various pathologic conditions, such as lymphadenopathy, tumors associated with surrounding organs (e.g., pancreas, adrenal, kidney), abdominal and pelvic masses, endometriosis, and metastases. Rarely, splenunculi may be manifested with nonspecific abdominal pain or become symptomatic when they undergo torsion, cyst formation, or hemorrhage.
Radiologic Findings
On ultrasound, accessory spleens are well-circumscribed, round or oval nodules with an echogenicity identical or slightly hypoechoic to that of the adjacent spleen ( Fig. 105-3A ). Seventy-five percent demonstrate acoustic enhancement or an incomplete hyperechoic rim, or both.

MR images of accessory spleens demonstrate signal intensity and enhancement similar to those of the normal spleen on all sequences ( Fig. 105-3B ). On CT, accessory spleens are well-marginated, rounded, homogeneously enhancing lesions ( Fig. 105-3C ). Attenuation of small accessory spleens (<1 cm) is often somewhat less than that of the spleen because of partial volume effects.
Heat-denatured red blood cell scintigraphy is considered the best study for detection of an accessory spleen because of its high sensitivity and specificity. Denatured red blood cells are labeled with technetium Tc 99m pertechnetate, after which single photon emission CT imaging is performed. On this examination, the round to ovoid foci with avid tracer uptake are seen colocating to the nodules seen on CT.
99m Tc-sulfur colloid scans are less commonly used to evaluate splenic disorders, but they are less accurate than heat-denatured red blood cell scans in the detection of splenunculi. Radioimmunoscintigraphy of the spleen provides no additional diagnostic information over heat-denatured red blood cell scintigraphy of the spleen in detecting splenunculi.
Differential Diagnosis
The differential diagnosis of supernumerary spleens includes lymphadenopathy, abdominal and pelvic masses, endometriosis, masses associated with surrounding organs (e.g., pancreas, adrenal, kidney), peritoneal mesothelioma, and metastases. Imaging characteristics similar to those of the normal spleen and heat-denatured red blood cell scintigraphy permit differentiation from other disorders.
Hemangioma
Epidemiology and Pathogenesis
Hemangioma is the most common benign neoplasm of the spleen, found in approximately 0.3% to 14% of autopsy specimens. Hemangiomas primarily affect young to middle-aged adults, but they are found in all age groups. Some investigators report predominance in males, whereas others did not find a gender predilection. Hemangiomas typically are found incidentally on radiologic imaging or pathologic studies. On occasion, they are multiple or diffuse, a condition called hemangiomatosis, or they are part of generalized angiomatosis, such as Klippel-Trénaunay-Weber syndrome.
Clinical Findings
Patients with hemangiomas are generally asymptomatic. Rarely, large hemangiomas or hemangiomatosis may be manifested with a palpable mass in the left upper quadrant, pain, or splenomegaly. Kasabach-Merritt syndrome, characterized by hemangiomatosis, thrombocytopenia, and intravascular coagulation, is a rare syndrome resulting from sequestration of red blood cells and platelets and consumption of clotting factors in the hemangiomas typically seen in early infancy. Spontaneous splenic rupture of splenic hemangiomas is extremely uncommon. Resection should be reserved for the rare situations, such as untreatable pain, diagnostic uncertainty, or compression of adjacent organs.
Pathology
On gross examination, hemangiomas appear blue to red and spongy, and they are typically well circumscribed. Most splenic hemangiomas are cavernous and a few are capillary or may demonstrate both features. On histopathologic examination, they are nonencapsulated proliferations of vascular channels of various sizes lined by a single layer of plump endothelial cells with little intervening fibrous tissue filled with red blood cells. Small hemangiomas of the spleen are typically homogeneously solid, although larger ones may demonstrate multiple cystic spaces of various sizes. They may contain calcifications. Diffuse hemangiomatosis is a rare, benign neoplastic condition in which the entire spleen is replaced by neoplastic blood vessels interspersed with sparse connective tissue.
Radiologic Findings
The imaging appearance of splenic hemangiomas depends on the gross morphology and is a spectrum from solid to mixed to purely cystic. Findings on abdominal radiographs are usually normal, but a large hemangioma may be manifested as a soft tissue mass in the left upper quadrant. If calcifications are present, they can be punctate or curvilinear.
On ultrasound, small splenic hemangiomas appear as discrete echogenic lesions similar to those in the liver ( Fig. 105-4 ). Large hemangiomas may appear as complex masses with both solid and cystic areas. Acoustic shadowing due to calcifications may be seen.

Hemangiomas may be manifested as solid, homogeneous masses or complex cystic masses with slight hypodensity on nonenhanced CT scans. Punctate, peripheral, and curvilinear calcifications may be present. Other splenic hemangiomas may appear cystic or heterogeneous, depending on the extent of the cystic and solid components. On contrast-enhanced CT, hemangiomas of the spleen may demonstrate a progressive centripetal enhancement pattern and become isodense on the delayed images, as can be seen in liver hemangiomas ( Fig. 105-5A, B ) although this occurs less frequently in the splenic hemangiomas than with hepatic hemangiomas. This probably reflects the differences in vascular supply of the liver and spleen.

On MR studies, splenic hemangiomas are isointense to hypointense on the T1-weighted images and typically hyperintense on the T2-weighted images compared with the normal spleen ( Fig. 105-5C ). Ramani and coworkers demonstrated in a study of 22 hemangiomas that 19 were hyperintense, two were isointense, and one was hypointense relative to the spleen on T2-weighted images. In the same study, a progressive centripetal pattern of enhancement was observed in 19 of 22 hemangiomas on the dynamic gadolinium-enhanced series, and 19 hemangiomas demonstrated uniform enhancement on the delayed images. Other types of enhancement that can be observed are immediate homogeneous and persistent enhancement on the delayed images and a peripheral centripetal enhancement with a persistent lack of central enhancement on delayed images. Sometimes, a central fibrous scar may demonstrate persistent enhancement. Cavernous hemangiomas typically demonstrate heterogeneous enhancement; the cystic components do not demonstrate enhancement on contrast-enhanced CT and MRI.
There is a paucity of published data regarding scintigraphy of splenic hemangiomas, and the results are often based on criteria used for the liver. 99m Tc-sulfur colloid scintigraphy of the liver and spleen may demonstrate a photopenic defect in the spleen. 99m Tc-labeled red blood cell scans demonstrate a hypovascular area on the blood flow images; early blood pool images may reveal a hypovascular lesion or demonstrate some pooling of tracer activity. Typically, the hemangiomas demonstrate increased activity on the delayed images, giving a perfusion–blood pool mismatch. This phenomenon is considered pathognomonic of hepatic hemangiomas.
Angiographic findings of splenic hemangiomas are variable and nonspecific. They can be hypovascular or hypervascular, with or without pooling of contrast material and with or without abnormal tumor vessels.
Hamartoma
Epidemiology and Pathogenesis
Hamartoma of the spleen is a rare, benign tumor first described by Rokitansky in 1859 as a splenoma. Hamartomas are also known as a spleen within a spleen, post-traumatic scars, nodular hyperplasia, and hyperplastic nodules. They are usually discovered incidentally during diagnostic imaging, splenectomy, or autopsy. Most are described in case reports or small series. The reported incidence in large autopsy and splenectomy series varies from 0.12% to 0.17%. The definitive diagnosis of a splenic hamartoma is rarely made on the basis of imaging. Hamartomas are often confirmed by pathologic evaluation after splenectomy.
No gender predilection has been demonstrated for hamartomas, and they can be found in any age group. Most hamartomas are solitary. Hamartomas of the spleen have been associated with hematologic disorders, tuberous sclerosis, malignant neoplasms, and Wiskott-Aldrich–like syndrome.
Clinical Findings
Hamartomas are usually discovered incidentally, and most patients are asymptomatic, but a large hamartoma may be manifested as a palpable mass or splenomegaly. Rupture of a splenic hamartoma is rare. Splenic hamartomas can be associated with hematologic disorders, including pancytopenia, anemia, and thrombocytopenia. This association probably results from sequestration of the hematopoietic cells in the abnormal vascular spaces. Resolution of these hematologic disorders after hamartoma resection has been reported. Associated growth retardation and recurrent infections have been reported in the pediatric population. It can be difficult, especially by cytologic analysis, to differentiate a hamartoma from a malignant neoplasm. Resection—partial or total splenectomy—is often performed because of diagnostic uncertainty.
Pathology
On gross examination, hamartomas are well-circumscribed, solid lesions that compress the surrounding parenchyma. They vary in size from less than 1 cm to more than 20 cm in diameter. On histologic examination, splenic hamartomas are composed of disorganized red pulp elements with reticuloendothelial cell proliferation. Normal white pulp is usually absent. They contain a mixture of unorganized sinus-like structures lined by endothelial cells surrounded by fibrotic cords of predominant splenic red pulp without a true capsule. Cystic spaces and coarse calcifications have been reported. Special immunohistochemical techniques can be used to differentiate hamartomas from hemangiomas. The etiology of splenic hamartomas is controversial; some authors consider splenic hamartomas to be congenital in origin, whereas others consider hamartomas to be true neoplasms or to be an acquired proliferative process.
Radiologic Findings
On sonography, hamartomas are typically well-defined, homogeneous masses ( Fig. 105-6A ). The reported appearance varies from isoechoic to mildly hyperechoic or hypoechoic. The mass may contain cystic areas or coarse calcifications. On color Doppler images, the mass often demonstrates increased blood flow. Some investigators report that sonography is more sensitive than CT in depicting hamartomas of the spleen.

A contour abnormality is often the only visible finding on CT ( Fig. 105-6B ). A typical hamartoma is isodense or mildly hypodense on nonenhanced and on intravenously enhanced CT scans ( Fig. 105-6C ). Heterogeneous enhancement can be observed in larger lesions; uniform and prolonged contrast enhancement is often shown on the delayed images.
On MRI, hamartomas are well-defined, isointense masses on the T1-weighted images. In a series of five patients, four were imaged with T2 sequences. In three of these, the lesions were heterogeneously hyperintense, and in one, the mass was hypointense relative to the normal spleen. In the same series, all hamartomas demonstrated diffuse, heterogeneous enhancement in the early phases and became more uniformly enhanced on delayed images on dynamic contrast-enhanced sequences. Similar MRI characteristics were described by other investigators, including prolonged enhancement on the delayed images after intravenous administration of gadolinium.
Angiography, sometimes performed for preoperative embolization, may demonstrate a hypervascular mass with peripheral tumor vessels and tumor stains.
The literature describing scintigraphic imaging findings for splenic hamartomas is sparse. Case reports of splenic hamartomas describe hot spots on 99m Tc-sulfur colloid scintigraphy, presumably caused by uptake in reticuloendothelial cells. The dynamic images of radiocolloid scans may show increased flow in the spleen. Other studies have described decreased uptake of the radiocolloid tracer compared with the normal spleen.
Although splenic hamartoma may be suspected on the basis of the imaging characteristics, a definitive diagnosis can rarely be made by the imaging findings alone. A definitive diagnosis may be established with a percutaneous biopsy, although this often proves to be a challenge for the pathologist, and more often, splenectomy is needed for a definitive diagnosis.
Lymphangioma
Epidemiology and Pathogenesis
Splenic lymphangiomas are rare, benign, slow-growing, congenital neoplasms. They are typically seen in childhood, with only a few reported cases in adults. Splenic lymphangiomas can be isolated solitary lesions within the spleen, or the entire spleen may be replaced (i.e., splenic lymphangiomatosis). This may be part of a rare congenital malformation of the lymphatics called lymphangiomatosis. Lymphangiomatosis predominantly affects pediatric patients and typically involves multiple organs and locations, including the neck, mediastinum, and retroperitoneum. Lymphangiomas of the spleen have also been associated with Klippel-Trénaunay-Weber syndrome.
Clinical Findings
In adults, splenic lymphangiomas are often asymptomatic. When symptoms are present, they are usually related to the increased size of the spleen and include left upper quadrant pain, nausea, and abdominal distention. Associated coagulopathy, hypersplenism, and portal hypertension have been described. Physical examination may reveal a tender mass in the left upper quadrant.
Management varies from conservative treatment for small, asymptomatic lesions to partial or total splenectomy for large, symptomatic lesions. Subtotal splenic embolization is also described as an effective treatment option.
Pathology
On gross examination, splenic lymphangiomas typically are subcapsular, multicystic lesions filled with watery pink proteinaceous fluid. On histologic evaluation, they are composed of multiple, thin-walled cysts of various sizes lined with a flat endothelium. Lymphangiomas often involve the capsule and trabeculae, where lymphatic structures are normally present. Lymphangiomas are traditionally classified as capillary, cavernous, or cystic, depending on the histologic findings.
Radiologic Findings
Findings on plain radiographs are usually normal, but they may demonstrate splenomegaly with a mass effect on adjacent viscera. Curvilinear calcifications can be present.
Ultrasound typically shows multiple cysts that are a few millimeters to several centimeters in diameter and are divided by thin septations ( Fig. 105-7 ). Splenic lymphangiomas may have calcifications, and the finding of internal echoes depends on the presence of proteinaceous or hemorrhagic fluid. Vascularity within the walls and the septa can be demonstrated with color Doppler imaging.

CT can reveal subcapsular, low-density, nonenhancing, sharply marginated, thin-walled cysts. Small, curvilinear or punctate calcifications may be observed. After administration of an intravenous contrast agent, the septa may demonstrate enhancement.
MRI reveals multiple, well-defined cysts within the spleen. They appear hypointense on T1-weighted images and hyperintense on the T2-weighted sequences. The cysts may demonstrate high signal intensity on the T1-weighted images if they contain proteinaceous or hemorrhagic fluid. The septa appear as hypointense structures on the T2-weighted sequences. These septa are usually better defined on contrast-enhanced CT and MR images.
When the lymphatic spaces are small, as in capillary lymphangiomas, the lesion may have a solid appearance on radiologic imaging, making a correct preoperative radiologic diagnosis difficult. The literature on angiographic and scintigraphic findings in splenic lymphangiomas is limited. Lymphangiomas do not demonstrate uptake of the radiopharmaceutical on 99m Tc-sulfur colloid scintigraphy.
On angiography, splenic lymphangiomas have been observed as round or oval, hypovascular mass lesions displacing the surrounding arterial branches without neovascularity. With more extensive involvement, many focal lucencies of various sizes can be seen in the venous phase, producing the so-called Swiss cheese appearance.
Inflammatory Pseudotumor
Epidemiology and Pathogenesis
Splenic inflammatory pseudotumor is a rare, benign lesion. Although the cause and pathogenesis are unknown, it is thought that inflammatory pseudotumors result from an unusual, nonspecific, inflammatory reparative response to injury such as infection.
Clinical Findings
Like most other benign splenic tumors, inflammatory pseudotumors are usually asymptomatic. In the typical scenario, this lesion is discovered incidentally, sometimes presumed to represent lymphoma. Fever and leukocytosis are the most commonly described associated symptoms. If the pseudotumor is large, a palpable mass may be discovered on physical examination.
Pathology
On gross examination, the lesions are often well defined and white. On histopathologic examination, the lesions are found to be well circumscribed and composed of spindle cells and various proportions of mixed inflammatory cells, including small lymphocytes, plasma cells, and histiocytes. A zonal distribution is characteristic, with central foci of necrosis and hemorrhage, foamy histiocytes, granulomas, and giant cells. Calcifications can be present.
Radiologic Findings
Unfortunately, radiologic imaging techniques do not permit preoperative diagnosis of these lesions. Splenic inflammatory pseudotumors are typically misdiagnosed as other masses, such as lymphoma or metastatic disease.
Abdominal radiographs are of limited utility. Ultrasound usually demonstrates a well-defined hypoechoic mass and acoustic shadowing when calcifications are present.
Unenhanced CT scans reveal a rounded mass with low attenuation with or without calcifications. Enhancement can be homogeneous or heterogeneous, and delayed enhancement patterns are often described. Central, stellate, low-density areas may persist within the mass, probably corresponding to focal areas of fibrosis.
On MRI, these lesions appear isointense to the splenic parenchyma on T1-weighted images. Both heterogeneously hypointense and hyperintense lesions compared with normal spleen tissue have been described on T2-weighted images. As with CT, most investigators describe a peripheral enhancement pattern with gradual, delayed central enhancement from the periphery to the center on the dynamic sequences.
Peliosis of the Spleen
Epidemiology and Pathogenesis
Splenic peliosis is a rare condition characterized by sinusoidal dilation and formation of multiple, cystlike, blood-filled cavities within the parenchyma. This condition most commonly affects the liver, but any organ, including the spleen, can be affected. Splenic peliosis as an isolated finding is rare; it is usually associated with hepatic peliosis. The pathogenesis of splenic peliosis is unclear. Splenic peliosis has been associated with various conditions, including infection, hematologic malignant neoplasms, use of anabolic steroids, and immunocompromised states.
Clinical Findings
Patients with splenic peliosis are often asymptomatic, and the condition is found incidentally on imaging studies or at autopsy. However, peliosis can be manifested with spontaneous splenic rupture. The first well-documented case of peliosis was reported in 1866, when Cohnheim described a spontaneous splenic rupture in a young man with peliosis.
Pathology
On gross examination, the size of the spleen is often found to be normal. Multiple, blood-filled cavities are round to oval and are 1 mm to several centimeters in diameter. The blood-filled cystic spaces may be arranged sporadically, may occur in clusters, or may be disseminated throughout the spleen. Thrombosis may occur in the cystic spaces. On microscopic examination, the cysts located within the red pulp are lined with flattened, sinusoidal endothelium, or they may lack a clear cell lining.
Radiologic Findings
No large series of splenic peliosis are available. The imaging findings are typically described in publications of hepatic peliosis and in case reports. The imaging findings of peliosis are variable and depend on the size of the cysts and whether there is thrombosis of the cystic contents.
Sonography typically reveals multiple, poorly defined, hypoechoic lesions. The lesions may be hyperechoic if thrombosis is present.
On unenhanced CT, peliosis lesions usually appear as multiple areas of low attenuation. On contrast-enhanced CT scans, splenic peliosis most commonly is manifested as multiple, small, well-defined, hypoattenuating, cystlike lesions. High-attenuation lesions and fluid-fluid levels, which are thought to reflect a hematocrit effect, demonstrating enhancement in their dependent portions have also been described.
The signal intensities of the lesions on MRI depend on the age and status of the blood components. On T2-weighted sequences, these lesions are usually hyperintense. On T1-weighted sequences, the lesions are typically hypointense or isointense to the normal spleen. After gadolinium injection, peliotic lesions may or may not enhance. Various patterns of enhancement have been described.
Angiography may reveal areas of pooling of contrast material and distortion of the intrasplenic vessels. If the lesions rupture, perisplenic hematoma, splenic laceration, and intraperitoneal hemorrhage may be evident.
Cystic Splenic Lesions
Cystic lesions of the spleen are relatively uncommon compared with the frequency with which cysts are encountered in other solid abdominal organs. The reported incidence of splenic cysts at autopsy is approximately 7.6 per 10,000 people. The true incidence is probably higher; in one series, the incidence was approximately 1% detected with abdominal ultrasound.
A wide variety of lesions may be manifested as splenic cysts ( Table 105-1 ). In Western countries, most splenic cysts are asymptomatic and discovered incidentally during routine abdominal imaging or autopsy. The most common splenic cysts in the Western world are congenital and traumatic in origin. Worldwide, parasitic cysts (most are echinococcal) are much more common and account for approximately 70% of splenic cysts. The correct cause and pathophysiologic mechanism of a splenic cyst can often be suggested by the medical history, symptoms, and appearance of the cyst on imaging.
| Type of Lesion | Examples |
|---|---|
| Congenital | Epidermoid cysts (primary, true, mesothelial) Dermoid (rare) |
| Acquired | Pseudocysts (secondary cysts): postinflammatory, post-traumatic, postinfarction, pancreatic |
| Infection | Parasitic: echinococcosis Abscess: pyogenic, fungal |
| Neoplastic | Benign: cystic hemangioma, cystic hamartoma, cystic lymphangioma, peliosis Malignant: angiosarcoma, lymphoma, metastases |
Congenital Cysts and Pseudocysts
Epidemiology and Pathogenesis
Traditionally, nonparasitic, non-neoplastic splenic cysts have been grouped as true cysts and pseudocysts on the basis of a classification system developed in 1940 by Fowler. Congenital cysts, also known as true, primary, epidermoid, or mesothelial cysts, have an epithelial or mesothelial cell lining and are thought to be developmental in origin. Pseudocysts, also known as secondary cysts or false cysts, do not have an epithelial lining.
Congenital cysts are believed to be the result of embryonic inclusions of splenic capsular mesothelial cells into the splenic parenchyma with subsequent gradual growth, although numerous other theories have been postulated. Congenital cysts account for approximately 10% of all splenic cysts, and they are not associated with cysts in other organs. Congenital cysts are often discovered in children and young adults, but they are found in all age groups, and they have a female predominance. A rare familial occurrence of splenic epidermoid cysts has also been reported. Dermoid cysts of the spleen are rare and have been described in only a few case reports.
Pseudocysts are thought to be the result of an intrasplenic hematoma, infarct, or infection, although a history of trauma or infection is often not apparent. They are more common in females, often discovered in young adults, but they can be found in all age groups. Most primary or secondary splenic cysts are located in the superior portion, but they can be found anywhere in the spleen.
Although Fowler’s categorization of splenic cysts is widely accepted, Morgenstern has made a case against the traditional classification of true and false cysts and argued that it is much more likely that most cysts are congenital in origin. He reasoned that a history of significant trauma is rarely obtained and that hemorrhagic cystic lesions after a known trauma usually have a different appearance. With the current practice of nonoperative management of splenic injuries, there should have been a significant rise in the incidence of splenic cysts. He stated that it can be difficult to identify an epithelial lining in splenic cysts and that this probably led to the erroneous classification of many reported cysts as pseudocysts.
The natural history of splenic cysts is unknown. No long-term follow-up reports of patients with splenic cysts have been published.
Clinical Findings
In Western countries, virtually all splenic cysts are asymptomatic and found during routine imaging. Symptoms may occur when the cysts are large and include left upper quadrant discomfort, fullness, and pain. Complications, such as spontaneous hemorrhage, rupture, or secondary infection, are rare. If the cyst is large, physical examination may reveal splenomegaly or a palpable mass with or without tenderness. Results of routine laboratory tests usually are normal, although epidermoid cysts of the spleen have been associated with elevated levels of cancer antigen 19-9 and carcinoembryonic antigen.
In our opinion, small and asymptomatic cysts are best managed conservatively. Treatment of large and symptomatic cysts includes percutaneous aspiration with or without sclerotherapy and partial splenectomy.
Pathology
In the series of Dachman and colleagues, true and false cysts could not be differentiated on the basis of gross appearance. True cysts are typically unilocular, and they have a trabeculated, shiny, pearly white lining. The fluid may be clear or turbid, and the color can be yellow, green, or brown, containing cholesterol or blood degradation products. On microscopic examination, the epithelial lining is variable, and a stratified squamous epithelium is the most common finding. On occasion, septations or thick, partially calcified walls can be seen. When inflammation or hemorrhage occurs in these cysts, the cellular lining may be replaced by nonspecific fibrous or granulation tissue, and histologic distinction between epidermoid cysts and pseudocysts becomes difficult.
Most pseudocysts are unilocular and have a smooth inner surface. The wall is formed by fibrous tissue with or without calcified components, and these cysts are often filled with serous or hemorrhagic fluid.
Radiologic Findings
It is not possible to distinguish between true and false cysts with any imaging technique. The wall of a pseudocyst tends to be thicker. If a cyst is large, it may be identified on plain radiographs. In certain instances, it may be possible to differentiate a cyst from splenomegaly by the observation of a round mass with a normal, intact splenic tip. Curvilinear calcifications may also help identify the presence of a splenic cyst ( Fig. 105-8 ).

On ultrasound, splenic cysts, like cysts elsewhere in the body, characteristically appear as anechoic lesions with smooth borders and demonstrate increased through-transmission ( Fig. 105-9A ). Epidermoid cysts and pseudocysts may demonstrate septations, thick walls, trabeculations, internal echoes, and calcified walls.

On CT, splenic cysts appear as homogeneous, well-defined, round or oval, low-enhancing or nonenhancing, hypodense lesions ( Fig. 105-9B ). The attenuation values typically range from 0 to 15 HU. Higher attenuation values are found in the presence of protein or blood degradation products. The cyst wall can be thick or partially calcified, and septations are sometimes observed.
On MRI, splenic cysts demonstrate low signal intensity on the T1-weighted images and high, uniform signal intensity on the T2-weighted images ( Fig. 105-9C ). Complicated cysts, because of the presence of proteinaceous fluid or hemorrhage, may demonstrate high signal intensity on the T1-weighted images and mixed signal intensity on the T2-weighted images. Cysts should not demonstrate any enhancement after the administration of gadolinium.
On scintigraphy, if the cyst is large enough, a solitary photopenic defect may be seen. Splenic cysts are avascular on angiography.
Hydatid Disease
Epidemiology and Pathogenesis
Hydatid disease, or echinococcosis, is a zoonotic tapeworm infection caused by the species Echinococcus granulosus . The larva, known as the hydatid, forms a cyst as it develops in the lung, liver, kidney, or spleen. The disease involves the spleen in approximately 2.5% to 6% of patients with abdominal hydatidosis and typically occurs in patients living in or emigrated from endemic areas. Splenic hydatid involvement is usually part of more widespread abdominal disease after rupture of a hydatid liver cyst. Primary echinococcal involvement of the spleen is rare. Alveolar echinococcal disease is caused by Echinococcus multilocularis and rarely involves the spleen.
Pathology
The hydatid cyst wall has three layers. The larvae of the tapeworm incite an inflammatory host response that forms an outer layer of inflammatory cells and fibrous tissue called the pericyst. The true cyst wall, also called the endocyst, is composed of an outer laminated membrane lined by an inner germinal layer made up of daughter cysts, also called the brood capsules. Scolices develop on the inner aspect of the brood capsules and after separation of the cyst wall form a sandlike material within the cysts. When the parasites die, the cysts become inactive, and the cysts may calcify and undergo fibrosis.
Clinical Findings
Splenic hydatidosis is often asymptomatic. Symptoms, if present, include abdominal pain and splenomegaly. More often, the clinical and radiologic findings are nonspecific, and the diagnosis should be suspected in all patients from endemic areas presenting with a splenic cyst.
Large cysts may cause compression of surrounding structures. Complications include infection, cyst rupture, and fistulization to surrounding organs. Rupture into the abdominal cavity may result in an anaphylactic reaction and the spread of multiple cysts throughout the abdomen. Serologic tests have a sensitivity of approximately 90% in diagnosis of hydatid disease. The imaging findings, combined with the clinical, serologic, and epidemiologic results, usually provide the correct diagnosis.
Albendazole is the mainstay of echinococcal treatment. Splenic hydatid cysts can be treated percutaneously with cyst aspiration and injection of hypertonic saline solution or alcohol, also known as the PAIR technique (i.e., puncture, aspiration, injection, reaspiration). Splenectomy or partial splenectomy can be performed if other treatment options have failed. Inactive cysts are typically observed and managed conservatively.
Classification
A World Health Organization Working Group on echinococcosis formulated a standardized sonographic classification of echinococcal cysts ( Table 105-2 ).
| Classification | Description |
|---|---|
| CL | Simple cystic lesion; no cyst wall visible |
| CE 1 | Unilocular simple cyst with visible cyst wall; may exhibit “hydatid sand,” fine internal echoes due to shifting of the brood capsules (i.e., snowflake sign) |
| CE 2 | Multivesicular, multiseptate cyst; daughter cysts may partially or completely fill the mother cyst; septations may produce wheel-like structures; contained daughter cysts may produce a rosette-like or honeycomb-like structure |
| CE 3 | Unilocular, anechoic content with detachment of the laminated membrane from the cyst wall, visible as a floating membrane (i.e., water lily sign), or complex masses caused by unilocular cysts containing daughter cysts and echoic areas |
| CE 4 | Heterogeneous hypoechoic or inhomogeneous degenerative contents; no daughter cysts; degenerating membranes may produce a ball of wool sign |
| CE 5 | Cysts with a thick, calcified, arch-shaped wall; lesions are partially or completely calcified |
The classification is intended to follow the natural history of cystic hydatid disease. Type CL cysts are usually seen at an early stage of development and are not fertile. These cysts cannot be differentiated from nonparasitic splenic cysts on the basis of imaging. Because the origin is uncertain, this cyst is not given the designation of a cystic echinococcosis (CE) type of lesion and is recorded as a cystic lesion (CL). The CE 1 and CE 2 types are active, usually fertile cysts containing viable protoscoleces. CE 3 cysts are degenerative and are entering a transitional stage. Most CE 4 and CE 5 cysts are inactive, nonfertile cysts.
Radiologic Imaging
The radiologic appearance of hydatid cysts reflects the natural history of the disease, in which daughter cyst formation is part of the natural aging process. The imaging findings are variable and range from purely cystic to solid-appearing pseudotumors, depending on the stage of the disease (see Table 105-2 ). All of these characteristic imaging appearances are lost when the cysts become infected.
Plain radiographs are of limited utility. Curvilinear or ringlike calcifications, representing calcifications of pericysts, could suggest cystic echinococcosis, especially in patients from endemic areas.
Ultrasound is the most popular and readily available technique worldwide for evaluation of abdominal echinococcal disease. Ultrasound is used for diagnosis, therapeutic decision-making, and follow-up. Portable ultrasound is also used in remote areas to screen, to diagnose, and to treat patients.
Fine internal echoes within the cysts may be seen because of shifting of the brood capsules, often called hydatid sand (i.e., the snowflake sign). Wavy bands of detached, laminated endocysts (i.e., the water lily sign) may be observed within the cysts. Daughter cysts may partially or completely fill the mother cyst ( Fig. 105-10A ). Calcifications, varying from tiny to massive, are often present.

CT and MRI are indicated for patients not suited for ultrasound and for evaluation of widespread or complicated disease. Cross-sectional imaging is helpful in planning percutaneous therapy or surgery. Subtle cyst wall calcifications are best demonstrated with CT.
On CT, a hydatid cyst may be manifested as a well-defined, hypoattenuating cystic lesion with water-attenuation values and a distinguishable wall. Coarse wall calcifications are often present. The hydatid cysts can be hyperdense because of debris, hydatid sand, and inflammatory cells. In CE 2 cysts, the round daughter cysts are arranged at the periphery, or they completely fill the mother cysts. CT attenuation of the daughter cysts is typically lower than that of the mother cysts. Type CE 3 lesions appear as relatively high attenuation, round or oval masses with scattered calcifications and occasional daughter cysts ( Fig. 105-10B ). Type CE 4 cysts may appear as a complex mass. The septa and cyst wall frequently enhance after the intravenous administration of contrast material. Type CE 5 cysts are complex cystic or solid-looking lesions, and they can be partially or completely calcified.
On MRI, the simple cysts are hypointense on the T1-weighted images and markedly hyperintense on the T2-weighted images. A low signal intensity rim (i.e., the rim sign), which is more evident on T2-weighted MR images, has been described as characteristic of hydatidosis. The septa and cyst wall frequently enhance after the intravenous administration of gadolinium. The daughter cysts may appear hypointense or isointense relative to the maternal matrix on T1- and T2-weighted images. The collapsed parasitic membranes may reflect a serpent sign or snake sign. These membranes have low signal intensity on all sequences. Type CE 4 and CE 5 cysts usually are hypointense on T1- and T2-weighted images.
The presence of a cystic lesion in a patient from an endemic area with positive serologic test results most likely indicates hydatid disease. In almost all cases of splenic hydatid cystic disease, hydatid liver disease coexists. The presence of daughter cysts is probably the most helpful imaging feature.
Differential Diagnosis of Cystic Splenic Masses
The differential diagnosis of cystic splenic masses is extensive (see Box 105-2 ) and includes true or false splenic cysts, parasitic (hydatid) cysts, pseudocysts related to pancreatitis, pyogenic abscess, benign tumors of the spleen (e.g., hemangiomas, hamartomas, lymphangiomas, lymphoma), and metastatic tumors. The correct diagnosis can usually be suggested by an analysis of clinical findings and symptoms, the medical history, and the appearance of the cyst.
Splenic cysts (true or false) are the most common in Western countries. They are typically well defined, are of homogeneous water density, and have a thin or imperceptible wall. After infection or hemorrhage, the cysts become more complex.
The presence of a cystic lesion with wall calcification and daughter cysts in a patient from an endemic area should raise the suspicion of cystic echinococcosis. Most cases of splenic hydatid cystic disease have coexisting hydatid liver disease.
Patients with a splenic abscess usually are sick or septic, and they present with fever, left upper abdominal pain, and leukocytosis. A cystic splenic mass developing in a patient with pancreatitis is likely to be a pancreatic pseudocyst.
Benign splenic neoplasms (e.g., hemangioma, lymphangioma, hamartoma) may show single or multiple cystic spaces in the mass that rarely fulfill the criteria for a simple cyst. Splenic lymphoma may be manifested as ill-defined, hypoechoic masses with internal echoes on ultrasound or as low-density lesions with or without a thick rim on CT scans, mimicking a cystic lesion. Cystic metastases to the spleen have been reported from melanoma and adenocarcinoma of the breast and ovary, but they usually occur in the setting of widespread metastatic disease and typically have a complex appearance. Isolated splenic metastases are rare.
Splenic Infection and Inflammatory Disease
Inflammation and infection of the spleen are manifested in three distinct patterns: generalized splenomegaly without discerning focal lesions; solitary lesions; and a diffuse, miliary or macronodular pattern with or without splenomegaly. Generalized splenomegaly is a nonspecific finding and can be found in a variety of diseases, including infections and inflammatory diseases ( Box 105-3 ). Multiple splenic nodular lesions, usually smaller than 2 cm in diameter, are commonly associated with nonbacterial infections, such as fungal and granulomatous infections. An abscess may be manifested as a solitary lesion. The presence of gas in a lesion, although rare, is pathognomonic of a pyogenic infection.
Congestive splenomegaly
Portal hypertension
Splenic vein thrombosis
Congestive heart failure
Infectious and inflammatory diseases
Acute infections (e.g., infectious mononucleosis)
Chronic infections (e.g., miliary tuberculosis, malaria)
Sarcoidosis
Systemic lupus erythematosus
Hyperplastic splenomegaly (i.e., hypertrophy)
Hemolytic anemias (e.g., malaria, polycythemia vera)
Infiltrative splenomegaly
Lymphomas, leukemias
Metastases
Extramedullary hematopoiesis
Storage diseases (e.g., Gaucher’s disease, amyloidosis)
Splenic neoplasms
Lymphangiomatosis
Hemangiomatosis
Peliosis
Splenic Nodular Disease
Multiple, small, nodular splenic lesions are encountered in a wide variety of diseases ( Box 105-4 ). These consist of bacterial, fungal, and protozoal infections; granulomatous diseases, including mycobacterial infections; and sarcoidosis and malignant neoplasms, such as lymphoma and metastases. Many patients with multiple splenic nodules have an established diagnosis, such as lymphoma, metastases, or tuberculosis. In these cases, the nodules are presumed to represent the same disease, and pathologic confirmation is usually not necessary. Multiorgan involvement is typical, and similar changes are seen in the liver and other organs.
Fungal microabscesses
Bacterial microabscesses
Protozoal infections
Tuberculosis
Mycobacterium avium complex infection
Pneumocystis jiroveci pneumonia
Cat-scratch disease
Sarcoidosis
Gamna-Gandy bodies
Hodgkin’s lymphoma
Non-Hodgkin’s lymphoma
Metastases
If no diagnosis has been established and the splenic nodules are an isolated finding, the diagnosis can rarely be made on the imaging findings alone. The different causes are often manifested in a clinically similar fashion, typically with splenomegaly and fever. The history and clinical presentation may narrow the differential diagnosis. Additional investigations, such as laboratory tests (including tumor markers), tuberculosis testing, and bone marrow biopsies, may be indicated. When the underlying disease is successfully treated, the nodules usually resolve or become calcified granulomas.
The imaging characteristics of the various nodular conditions are nonspecific and similar. The nodules are often numerous and are a few millimeters up to 2 cm in diameter. They are hypoechoic on ultrasound and hypoattenuating on CT. The lesions appear hypointense on T1-weighted sequences and vary from hypointense to hyperintense on the T2-weighted sequences. The nodules usually do not enhance or may show ring enhancement after intravenous administration of contrast material. Healed granulomas appear as scattered, discrete, small calcifications in an otherwise normal spleen.
Fungal Abscess and Microabscess
Fungal infections of the spleen typically occur in immunocompromised patients with neutropenia. Acquired immunodeficiency syndrome (AIDS), chemotherapy, immunosuppressive agents, and lymphoproliferative disorders are the most common risk factors. Hepatosplenic fungal infection is found in approximately 7% of patients with acute leukemia, and these patients have a poor prognosis. Candida is the fungal organism most frequently encountered, followed by Aspergillus , Cryptococcus , and Histoplasma .
When the host response improves, the focus of candidiasis becomes encapsulated and walled off by neutrophils and inflammatory cells. As a result, the findings on diagnostic imaging are often normal in patients with chronic disseminated candidiasis because the characteristic changes become visible only when the neutrophil count recovers. In an analogous case, the phenomenon of waning of focal hepatic and splenic lesions on imaging during neutropenia was described by Pestalozzi.
No single imaging technique has proved to be specific and sensitive in patients with suspected or proven fungal infections, and only serial imaging may be able to detect hepatosplenic involvement. Regardless of the imaging findings, a biopsy may be necessary to establish the final diagnosis because blood cultures are often falsely negative, particularly with Candida infections.
Pathology
On histologic examination, these lesions are poorly confined, containing pseudohyphae and yeast forms. On microscopic examination, the lesions are multilayered, consisting of an outer ring of fibrosis, a middle area of inflammatory cells, and a central area of necrosis.
Clinical Findings
The most common manifestation of systemic candidiasis is persistent fever not responsive to conventional antibiotics. The clinical diagnosis of fungal infection is often difficult because the presenting symptoms may be similar to those of the patient’s primary disorder: fever and splenomegaly. Unexplained clinical deterioration in an immunosuppressed patient should raise the suspicion of a disseminated fungal infection.
Radiologic Findings
The limitation of all imaging modalities in the diagnosis of hepatosplenic fungal disease is the inability to visualize fungal lesions during the neutropenic phase. It is important to consider repeating the studies after recovery of the neutrophil count, especially if the clinical suspicion is high and the patient is not responding to conventional antibiotic therapy.
Different sonographic patterns of hepatosplenic candidiasis have been described. Multiple, small, hypoechoic nodules are the most common finding ( Fig. 105-11A ). A less common appearance can be seen in the early course of infection and has been described as the wheel-within-a-wheel appearance (i.e., the target sign). It is caused by a peripheral hypoechoic zone of fibrosis, the first wheel, and an echogenic second wheel of inflammatory cells around a central echogenic nidus containing necrosis and fungal elements ( Fig. 105-11B ). This type of lesion may evolve into a bull’s-eye lesion, which histologically corresponds to inflammatory cells surrounded by fibrosis. Late in the disease with healing, lesions usually become small and hyperechoic with various degrees of posterior acoustic shadowing, with or without calcification, or they may disappear. The use of higher frequency, linear array transducers significantly improves lesion detection.

On CT, fungal microabscesses are typically 5- to 10-mm, hypodense, nodular lesions ( Fig. 105-11C ). The detection rate is low (30%) on noncontrast scans. In the arterial phase (25-35 seconds), most lesions (70%) demonstrate an enhancing peripheral ring that typically disappears in the portal venous phase. In the study by Metser and associates, the detection rate of about 90% was not significantly different between the arterial phase and portal venous phase. Similar findings are typical for the liver and sometimes for the kidneys.
Rudolph and colleagues demonstrated another appearance in approximately 30% of patients. In the arterial phase, these lesions showed an inhomogeneous central enhancement surrounded by a double target ring consisting of a hypodense inner ring surrounded by a hyperdense outer ring. Over time, contrast enhancement is in a centrifugal direction, and the lesions appear smaller or disappear in the portal venous phase.
MRI is superior to CT and ultrasound for identification of hepatosplenic fungal disease, with a reported sensitivity of 100% and specificity of 96%. MRI is especially useful for following the course of infection during antifungal therapy and for evaluating the response to treatment.
On MRI, in the acute phase of hepatosplenic fungal disease, the lesions are small, measuring less than 1 cm in diameter. They are mildly hypointense on the T1-weighted images and markedly hyperintense on the T2-weighted images.
In the subacute phase, the lesions tend to be mildly hyperintense on both T1- and T2-weighted sequences. A peripheral ring of very low signal intensity is typical on all sequences in the subacute phase. The central region of the lesions may demonstrate enhancement after gadolinium administration, with the peripheral ring continuing to have low signal intensity, making them more visible. The time for transformation from acute to subacute lesions is 2 weeks to 3 months.
After successful antifungal treatment, the 1- to 3-cm lesions become irregular, and the central area disappears. The time to appearance of healed fungal foci on MRI ranges from 3 months to more than 1 year.
99m Tc-sulfur colloid liver-spleen, indium In 111–labeled leukocyte, and gallium Ga 67 scans usually are not helpful in detecting microabscesses because normal uptake of the radiotracer obscures the small lesions. Results of 67 Ga scans are variable and may show multifocal areas of increased or decreased uptake in the spleen.
FDG PET scans show increased 18 F-fluorodeoxyglucose uptake. This represents a promising imaging technique in patients at high risk for infections, although published data regarding fungal infections are still limited.
Tuberculosis
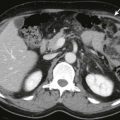
Mycobacterium tuberculosis is an important health problem in developing countries; in Western countries, it is typically seen in immunocompromised patients. Splenic tuberculosis usually occurs in the setting of disseminated, miliary infection and is manifested as multiple splenic nodules between 0.2 and 1 cm in diameter. Macronodular presentation is rare. In cases of disseminated, miliary pulmonary tuberculosis, splenic involvement has been found in 80% to 100% at autopsy. Associated findings include lymphadenopathy and a hypoechoic pattern on ultrasound or central low attenuation on CT, which is more commonly seen in patients with Mycobacterium avium complex infection ( Fig. 105-12 ).

Mycobacterium Avium Complex Infection
Mycobacterium avium complex infections of the spleen, also called Mycobacterium avium-intracellulare infections, are typically seen in immunocompromised patients, such as those with AIDS. Splenomegaly with multiple, low-attenuation nodules can be seen on imaging ( Fig. 105-13 ). Associated findings, such as marked hepatic and splenic enlargement, diffuse jejunal wall thickening, and enlarged lymph nodes, suggest disseminated M. avium complex infection. Patients with M. avium complex infection tend to have more homogeneous lymph nodes compared with those with tuberculosis, but a tissue diagnosis is mandatory.

Pneumocystis Jiroveci Pneumonia
Pneumocystis jiroveci pneumonia, formerly known as Pneumocystis carinii pneumonia, is the most common opportunistic infection in patients with AIDS. Extrapulmonary involvement causes necrotizing granulomas. Intrasplenic P. jiroveci infection is often discovered incidentally in patients with AIDS undergoing diagnostic imaging for a fever of unknown origin. When it is treated successfully, the nodules may enlarge and become progressively calcified in a rimlike or a punctate fashion. On ultrasound, the findings include splenomegaly with small, hypoechoic lesions with cystic components or tiny, highly reflective, nonshadowing foci or calcified granulomas ( Fig. 105-14 ). The nodules are hypodense on CT or manifested as calcified granulomas in the later stages of disease.

Cat-Scratch Disease
Cat-scratch disease is a self-limited infection by Bartonella henselae that occurs after being scratched by a domestic cat. It affects an estimated 22,000 people in the United States each year, and most are children and adolescents. Hepatosplenic involvement is uncommon, but B. henselae infection should be considered in a patient with fever of unknown origin, abdominal pain, and multiple hypodense lesions in the liver and spleen.
Sarcoidosis
Sarcoidosis is a systemic disease of unknown cause characterized by formation of noncaseating, epithelioid granulomas. Sarcoidosis most commonly affects the lungs and intrathoracic lymph nodes, but any organ system can be involved. Splenomegaly, typically associated with hepatomegaly and abdominal lymphadenopathy, is usually mild and seen in 25% to 60% of these patients. Massive splenomegaly (>18 cm long) is seen in approximately 6% of patients with sarcoidosis.
Histologic evidence of liver and spleen involvement is seen in up to 77% at autopsy, but it is a relatively uncommon finding at diagnostic imaging. On imaging, splenic nodules are seen in 6% to 33% of patients with sarcoid. In one study, these patients typically presented with hepatosplenomegaly, abdominal lymphadenopathy, and symptoms such as abdominal pain, fatigue, and malaise. In the same study, the nodules were not associated with advanced lung disease and did not indicate a change in chest radiographic stage. The infradiaphragmatic adenopathy in sarcoidosis tends to be small and discrete, and retrocrural adenopathy is uncommon in these patients compared with patients with lymphoma. There is, however, a significant overlap in appearance and distribution of the lymph nodes in lymphoma and sarcoidosis, and these criteria cannot be used for accurate disease characterization.
The nodules in sarcoid are 0.1 to 3.0 cm in diameter. On ultrasound, the nodules have been described as hypoechoic to slightly hyperechoic or inhomogeneous. On CT, the nodules are hypodense relative to adjacent normal spleen after intravenous administration of a contrast agent ( Fig. 105-15 ). The nodules are hypointense on all MR sequences and best seen on early-phase, gadolinium-enhanced, T2-weighted, fat-suppressed images or on T1-weighted sequences. The nodules do not enhance after intravenous administration of contrast material on CT or MRI.

Lymphoma or Metastatic Disease
Lymphomas—typically small cell lymphomas and mantle cell lymphomas and, less frequently, Hodgkin’s lymphoma—can be manifested with multiple, nodular splenic lesions or with a miliary pattern. Nodules resembling those found in sarcoidosis have been described in patients with Hodgkin’s lymphoma. This finding is usually part of disseminated disease including lymphadenopathy. Isolated splenic lymphoma is rare.
Metastatic disease to the spleen may be manifested as solitary or multiple nodules, rarely in a miliary pattern. This typically occurs in patients with advanced disease and widespread involvement of other organs.
Gamna-Gandy Bodies
Gamna-Gandy bodies are siderotic nodules caused by focal organized hemorrhagic infarcts. They are typically seen in patients with congestive splenomegaly and sickle cell disease. They were also described in patients with hemolytic anemia, leukemia, lymphoma, or acquired hemochromatosis and in patients receiving multiple blood transfusions. The siderotic nodules occur in 9% to 12% of patients with portal hypertension and are not related to the degree of splenomegaly. On histologic examination, the nodules are small, yellow or brown, fibrotic lesions containing hemosiderin and calcium and sometimes containing foreign body giant cells.
Gamna-Gandy bodies are best seen by MRI as multiple, 3- to 8-mm, markedly hypointense nodules on T1-weighted and T2-weighted images. The gradient recalled echo sequence is the most sensitive for detection of hemosiderin and these nodules. Nodule conspicuity increases after gadolinium enhancement on the T1-weighted, gradient recalled echo images ( Fig. 105-16 ). Gamna-Gandy bodies do not enhance.

Ultrasound is less accurate than MRI. In a study by Chan and associates, ultrasound detected the nodules in 24 of 34 patients, with a sensitivity of 70.6% and a specificity of 78.9%. Gamna-Gandy bodies appear as multiple, punctate, hyperechoic foci.
Unenhanced CT may detect Gamna-Gandy bodies as multiple, faint, hyperdense nodules shown by the calcifications within them. However, the nodules often are not detected on CT.
Pyogenic Abscess
Epidemiology and Pathogenesis
An isolated splenic abscess is a rare entity, with a reported incidence in autopsy series between 0.14% and 0.7%. However, splenic abscesses are becoming more common as a result of an increasing number of immunosuppressed patients, patients with AIDS, and injection drug abusers. Pyogenic splenic abscesses are usually solitary and unilocular or multilocular, but they can also be multifocal.
Most abscesses are caused by disseminated infection and are seen in patients with sepsis and septic emboli. Less common routes are contiguous infection (e.g., infected pancreatitis, perinephric abscess) and superinfection after an underlying splenic injury (e.g., trauma, infarct). In approximately 20% of cases, no source or cause is discovered. Roughly one third of patients have other concurrent extrasplenic abscesses. The reported mortality rate for splenic abscess in the literature is about 10%. Multiple splenic abscesses, gas-containing abscesses, and gram-negative bacillus infection are poor prognostic factors. Depending on the underlying disease, a variety of microorganisms may be encountered, including gram-negative bacilli (e.g., Klebsiella pneumoniae ), gram-positive cocci, fungi, polybacteria, and mycobacteria.
Clinical Findings
The most common clinical presentations in patients with splenic abscesses are fever (92%), left upper abdominal pain (77%), and leukocytosis (66%). Other symptoms include left pleural effusion and splenomegaly. Diabetes, immunosuppression, or immunodeficiency is often a contributing factor.
Early diagnosis of a splenic abscess with identification of organisms and determination of sensitivity is essential for effective antibiotic treatment. In small abscesses, percutaneous diagnostic aspiration can be performed. For larger abscesses, percutaneous drainage procedures have become the treatment of choice, allowing preservation of the spleen. Splenectomy is reserved for complicated cases. Complications of splenic abscesses include rupture and peritonitis with potential life-threatening results.
Radiologic Findings
The appearance of a splenic abscess depends on the stage of development. In the early phase, there is an ill-defined mass that later develops into a complex collection with septations and debris and that sometimes contains gas. When a capsule has developed, the lesion becomes well defined. Splenic abscesses can be solitary and unilocular, solitary and multilocular, or multifocal. The infecting organism cannot be predicted on the basis of imaging appearances.
The most frequent plain radiographic finding in splenic abscess is a left pleural effusion (42%), followed by an infiltrate in the left lung base (20%) and splenomegaly. A splenic abscess is rarely identified by extraluminal gas or air-fluid levels in the left upper quadrant on plain films.
Ultrasound has a sensitivity of 75% to 98% in detecting a splenic abscess. Sonography is especially useful as a screening examination for bedridden patients, for patients with renal impairment, and for evaluation of small splenic lesions. In some patients, the examination may be technically difficult because of overlying gas from the bowel or lung. The sonographic appearance of a splenic abscess depends on the stage of development, and a typical pattern is encountered in only 44% of cases. In the early phase, there is an ill-defined, hypoechoic lesion mimicking a mass. Later, the abscess may develop into an anechoic cysts or a complex cystic lesion with septa, debris, and acoustic shadowing caused by gas ( Fig. 105-17A ). When a capsule has developed, the lesion is typically well defined with a thin, hyperechoic rim.

On CT, a splenic abscess typically is manifested as a unilocular or multilocular, hypodense collection or complex cystic lesion with an enhancing rim after intravenous administration of contrast material ( Fig. 105-17B ). CT can be diagnostic if the collection contains gas, but this is a rare finding.
The advantages of CT are high sensitivity (92%-98%), noninvasiveness, and speed. CT aids in differentiating unilocular from multilocular abscesses, is excellent for exact localization, and provides better anatomic information about the perisplenic area. This information can then be used to guide further management ( Fig. 105-17C ). CT may reveal concurrent areas of infection and can help detect the underlying source of disease.
MRI is not often performed in the work-up of patients with splenic abscesses because CT is highly sensitive, and many patients are not clinically stable. Splenic abscesses are hypointense on the T1-weighted images and mildly to moderately hyperintense on the T2-weighted images compared with the normal spleen; they demonstrate minimal to intense peripheral enhancement after intravenous administration of gadolinium.
Nuclear medicine plays a limited role in the detection and localization of splenic abscesses. If the abscess is more than 2 cm in diameter, 99m Tc-sulfur colloid scans of the liver and spleen may demonstrate a nonspecific splenic filling defect. The 67 Ga scan and the 111 In-labeled leukocyte scan may show increased accumulation of radionuclide in the spleen. However, normal, inherent splenic activity on 67 Ga scans and 111 In-labeled leukocyte scans typically obscures an inflammatory focus within or near the spleen, resulting in a false-negative examination finding. A 67 Ga scan is nonspecific because tracer uptake is also seen in neoplastic lesions, particularly in lymphomas. A 99m Tc-sulfur colloid scan of the liver and spleen before injection of 111 In-labeled leukocytes appears to improve detection and characterization compared with performing 111 In-tagged leukocyte scans alone. FDG PET/CT is helpful in detecting a site of infection; however, its role in diagnosis of a focal splenic abscess has not been evaluated.
Angiography is sometimes used for management of vascular complications. Intrasplenic mycotic aneurysms are rare and described only in case reports. If angiography is performed, the abscess appears as a mass with attenuated vessels and a hypervascular rim.
Splenic Infarction
Epidemiology and Pathogenesis
Splenic infarction, the result of arterial or venous compromise, has many potential causes, including hematologic disorders, thromboembolic disease, vascular diseases, and trauma ( Box 105-5 ). Although splenic infarction is a relatively common finding, only a few larger series have been published, and most of the literature consists of case reports.
Embolic sources
Thromboembolic diseases
Septic emboli
Atherosclerosis
Therapeutic transcatheter embolization
Splenic sources
Massive splenomegaly
Hematologic diseases
Sickle cell disease
Myelofibrosis with myeloid metaplasia
Polycythemia vera
Neoplasms
Leukemia
Lymphoma
Vasculitis
Polyarteritis nodosa
Systemic lupus erythematosus
Rheumatoid arthritis (e.g., Felty’s syndrome)
Pancreatic disease
Pancreatitis
Storage disease
Gaucher’s disease
Amyloid
Splenic artery
Trauma
Acute splenic torsion
Pancreatic masses causing splenic arterial occlusion or extrinsic compression
Splenic vein
Portal hypertension
Splenic vein thrombosis
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree