In this article, the authors review the anatomy, pathophysiology, MR imaging features, and diagnostic criteria for benign uterine conditions, including adenomyosis, uterine leiomyomas, retained products of conception, and uterine arteriovenous malformations. Pearls, pitfalls, and variants are discussed for each entity as well as important imaging features that can affect management decisions.
Key points
- •
Adenomyosis is a common, frequently debilitating condition affecting women in their later reproductive years.
- •
Uterine leiomyomas are the most common tumor of the uterus, affecting up to 80% of women, and can be a cause of abnormal uterine bleeding, pelvic pain and mass effect, and reproductive or sexual dysfunction.
- •
Differentiating retained products of conception, a common cause of postpartum hemorrhage, from uterine arteriovenous malformation is important in order to allow for optimal treatment.
- •
Although ultrasound is the first line imaging modality in evaluation of the uterus, MR imaging has become a useful and reliable noninvasive tool in diagnosis and problem solving in all of these entities.
Background
Relevant Uterine Anatomy
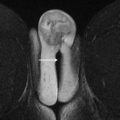
The anatomy of the normal, premenopausal uterus is traditionally divided into 2 distinct components: the endometrium and the myometrium. MR imaging has the advantage of further stratifying the anatomic layers into 3 well-depicted zones on T2-weighted imaging (T2WI), as follows ( Fig. 1 ):
- 1.
Endometrium : It has high signal on T2WI due to the presence of secretions and abundant cytoplasm. In reproductive-aged women, normal endometrial thickness varies between 3 and 6 mm in the proliferative phase and 5 and 13 mm in the secretory phase.
- 2.
Inner myometrium/junctional zone (JZ) : This superficial myometrial layer consists of myocytes with a high nuclear-to-cytoplasmic ratio and reduced water content, relative to the deeper myometrial myocytes. This unique morphology results in an intrinsic, lower signal on T2WI. The concentric orientation of smooth muscle fibers, in contrast to the longitudinal arrangement in the outer myometrium, also contributes to the lower signal of the JZ. The JZ is the presumed site of origin of uterine contractions and peristalsis.
- 3.
Outer myometrium : The outer myometrium consists of myocytes with a lower nuclear-to-cytoplasmic ratio. The relative increase in water content and the longitudinal orientation of smooth muscle fibers results in a higher T2 signal than the JZ.

Physiologic variation of the JZ may occur with age or with changes in hormone levels. In postmenopausal patients, there is progressive dehydration of the outer myometrium, which lowers the T2 signal and, therefore, the distinction between the JZ and outer myometrium. With increasing age, the smooth muscle of the myometrium undergoes progressive atrophy. Therefore, the JZ may not be delineated in up to 30% of postmenopausal patients.
Patient Preparation
Bladder emptying is recommended immediately before the examination in order to reduce artifacts related to bladder filling and patient discomfort. Motion artifacts related to small bowel peristalsis may be minimized using antiperistaltic agents, such as hyoscine butylbromide (20 mg intramuscularly [IM]/intravenously [IV]) or glucagon (0.5–1.0 mg IM/IV). Routine use of antiperistaltic agents in pelvic MR imaging has been shown to significantly improve image quality.
MR Imaging Protocol
Uterine evaluation is optimized using high-resolution, thin-section MR imaging with images acquired at 1.5 T or 3.0 T. Optimal signal-to-noise ratio may be achieved using a phased-array surface coil.
Recommended sequences include
- •
High-resolution, free-breathing T2 sequences in the axial, oblique, sagittal, and coronal planes is recommended. (High-resolution, 3-dimensional [3D], T2WI with multi-planar reformatting may also be used.) Sagittal T2WI best depicts the zonal anatomy of the uterus. T2WI acquisitions in more than one plane are helpful to avoid misinterpretation of transient uterine contractions.
- •
Axial T1-weighted sequence with and without fat suppression is recommended.
- •
Contrast-enhanced axial T1-weighted images (T1WI) of the pelvis with fat saturation is recommended.
Background
Relevant Uterine Anatomy
The anatomy of the normal, premenopausal uterus is traditionally divided into 2 distinct components: the endometrium and the myometrium. MR imaging has the advantage of further stratifying the anatomic layers into 3 well-depicted zones on T2-weighted imaging (T2WI), as follows ( Fig. 1 ):
- 1.
Endometrium : It has high signal on T2WI due to the presence of secretions and abundant cytoplasm. In reproductive-aged women, normal endometrial thickness varies between 3 and 6 mm in the proliferative phase and 5 and 13 mm in the secretory phase.
- 2.
Inner myometrium/junctional zone (JZ) : This superficial myometrial layer consists of myocytes with a high nuclear-to-cytoplasmic ratio and reduced water content, relative to the deeper myometrial myocytes. This unique morphology results in an intrinsic, lower signal on T2WI. The concentric orientation of smooth muscle fibers, in contrast to the longitudinal arrangement in the outer myometrium, also contributes to the lower signal of the JZ. The JZ is the presumed site of origin of uterine contractions and peristalsis.
- 3.
Outer myometrium : The outer myometrium consists of myocytes with a lower nuclear-to-cytoplasmic ratio. The relative increase in water content and the longitudinal orientation of smooth muscle fibers results in a higher T2 signal than the JZ.
Physiologic variation of the JZ may occur with age or with changes in hormone levels. In postmenopausal patients, there is progressive dehydration of the outer myometrium, which lowers the T2 signal and, therefore, the distinction between the JZ and outer myometrium. With increasing age, the smooth muscle of the myometrium undergoes progressive atrophy. Therefore, the JZ may not be delineated in up to 30% of postmenopausal patients.
Patient Preparation
Bladder emptying is recommended immediately before the examination in order to reduce artifacts related to bladder filling and patient discomfort. Motion artifacts related to small bowel peristalsis may be minimized using antiperistaltic agents, such as hyoscine butylbromide (20 mg intramuscularly [IM]/intravenously [IV]) or glucagon (0.5–1.0 mg IM/IV). Routine use of antiperistaltic agents in pelvic MR imaging has been shown to significantly improve image quality.
MR Imaging Protocol
Uterine evaluation is optimized using high-resolution, thin-section MR imaging with images acquired at 1.5 T or 3.0 T. Optimal signal-to-noise ratio may be achieved using a phased-array surface coil.
Recommended sequences include
- •
High-resolution, free-breathing T2 sequences in the axial, oblique, sagittal, and coronal planes is recommended. (High-resolution, 3-dimensional [3D], T2WI with multi-planar reformatting may also be used.) Sagittal T2WI best depicts the zonal anatomy of the uterus. T2WI acquisitions in more than one plane are helpful to avoid misinterpretation of transient uterine contractions.
- •
Axial T1-weighted sequence with and without fat suppression is recommended.
- •
Contrast-enhanced axial T1-weighted images (T1WI) of the pelvis with fat saturation is recommended.
Adenomyosis
Discussion of Problem/Clinical Presentation
- •
Adenomyosis of the uterus is a common gynecologic condition, affecting 1% of women in the fourth and fifth decades of life and 20% to 35% of women undergoing hysterectomy for benign gynecologic disorders. Most affected women are multiparous.
- •
The condition occurs at the endometrial-myometrial interface and is characterized by the presence of ectopic endometrial glands and stroma within the myometrium, with adjacent smooth muscle hyperplasia.
- •
Up to one-third of patients are asymptomatic. Symptomatic patients most frequently present with menorrhagia (50%), dysmenorrhea (30%), and metrorrhagia (20%). Occasionally, dyspareunia may be a presenting complaint.
- •
Adenomyosis is associated with female infertility due to the overlapping pathophysiology and association with endometriosis.
Pathophysiology
Adenomyosis is characterized by the ectopic, intramyometrial location of endometrial glands and stroma. The ectopic glands are surrounded by reactive hypertrophy of the myometrium, often leading to globular enlargement of the myometrium and uterus. Occasionally, extravasated blood products may accumulate within cystic intramyometrial spaces. At surgery, foci of ectopic endometrial tissue may be macroscopically visible on the serosal surface of a hysterectomy specimen ( Fig. 2 ). At the microscopic level, adenomyosis is arrayed randomly within the myometrium, usually at least 2 mm deep to the basal endometrial layer ( Fig. 3 ).
The pathogenesis of adenomyosis is unclear, but several mechanisms have been proposed :
- 1.
Uterine autotraumatization : This widely accepted theory suggests that chronic uterine peristaltic activity and hyperperistalsis induce microtraumatizations at the endometrial-myometrial interface. The endometrial/myometrial interface is structurally a point of weakness and is, therefore, susceptible to microtrauma, usually due to hyperperistalsis (iatrogenic microtrauma is also a potential inciting factor). This autotraumatization by peristaltic activity, in turn, activates a cycle of tissue injury and repair, resulting in local production of estrogen. The relative increase in local estrogen production by the uterus, which increases uterine peristalsis, competes with a function normally controlled by the ovaries. These interactions lead to a state of perpetual hyperperistalsis and a cyclical disease process. This theory is supported by evidence indicating that estradiol levels are elevated in the menstrual blood of women with adenomyosis.
- 2.
Impairment of repair mechanisms normally involving the basal endometrial layer : Endometrial stroma invaginates into the myometrium at structural points of weakness along the basal endometrial layer. This invagination may be due to focal disruption; however, absence of enzymatic activity required specifically to maintain the contiguity of the basal layer is also a possible cause.
- 3.
Lymphatic contamination : Invagination of the basal endometrial layer into the myometrium may occur via the local lymphatic channels, resulting in ectopic endometrial tissue.
- 4.
Metaplastic transformation : As Müllerian tissue is pluripotent, this theory proposes that myometrial tissue within the JZ differentiates into the glandular and stromal tissue characteristic of the endometrium.
Histologic examination of the myometrium in patients with and without adenomyosis suggests that a chronic state of hyperperistalsis may alter the myometrial ultrastructure, causing distortion of the myocytes, nuclear enlargement, and reduced prominence of collagen fibrils due to expansion of the extracellular space. Intracellular calcium is also increased in myocytes harboring adenomyosis, which has been shown to affect contractility. This dysfunctional contractility may manifest clinically as dysmenorrhea.
MR Imaging Features and Diagnostic Criteria
Studies evaluating the diagnostic performance of MR imaging in adenomyosis describe a sensitivity of 70% to 86%, specificity of 86% to 93%, and mean accuracy of 87.5%. In contrast, accuracy of clinical diagnosis alone ranges from 2.6% to 26.0%, due to the nonspecific clinical presentation.
Features of adenomyosis on MR imaging may be stratified into direct and indirect signs. Direct signs have specific correlation with the presence of endometrial glands within the myometrium, whereas indirect signs are the result of reactive changes in the myometrium ( Table 1 ).
| Direct Signs | Indirect Signs | MR Imaging Features |
|---|---|---|
| Microcysts | — | Intramyometrial cysts measuring 2–7 mm (average 3 mm); usually simple fluid signal on T1WI and T2WI, occasionally may have high signal on T1WI |
| Adenomyoma | — | Focal consolidation of ectopic endometrial glands, often distinct from JZ; often contain foci of high signal on T2WI |
| — | JZ thickening | Smooth muscle hypertrophy reactive to presence of ectopic endometrial tissue; >12 mm thickness |
| — | JZ differential | >5 mm differential between maximal and minimal uterine JZ thicknesses |
Direct Signs
- 1.
Submucosal microcysts : They are focal pools of fluid embedded within the myometrium, varying in size from 2 to 7 mm. These microcysts typically demonstrate water signal on T1WI and T2WI. While considered a specific sign of adenomyosis, they are detected in only 50% of cases. Occasionally, toward the end of the luteal phase, hemorrhagic content may accumulate within the cysts, manifesting as T1 shortening ( Fig. 4 ).
Fig. 4
Diffuse adenomyosis with hemorrhagic, submucosal microcysts. ( A ) Sagittal and ( B ) axial T2WI show diffusely thickened JZ ( arrow ) with microcysts ( arrowhead ) with intermediate to high T2 signal. Axial T1WI with fat saturation ( C ) image demonstrates corresponding high signal (hemorrhage) ( arrow ) within microcyst.
- 2.
Adenomyoma : Masslike consolidation of adenomyotic glands within the myometrium, appearing distinct from the JZ. The distinction from leiomyoma may be challenging; adenomyomas are usually low signal on T2WI but may contain punctate foci of high T2 signal. They do not typically have feeding vessels in the vicinity, in contrast to leiomyomas. Recognition of this entity is important for patient management, as myomectomy or polypectomy may be curative for leiomyomas but ineffective for adenomyomas ( Fig. 5 ).
Fig. 5
A 28-year-old woman with adenomyoma who presents with pelvic pain and menorrhagia. Sagittal single shot fast spin echo T2 weighted image (a), demonstrates focal thickening of the JZ ( arrows ) of the myometrium, with multiple small cystic spaces ( arrow ). Imaging findings are classic for adenomyosis. This case demonstrates focal adenomyosis/adenomyoma. Three arrows point to the area of adenomyoma/adenomyosis.
Indirect Signs
- 1.
Thickening of the JZ results from smooth muscle hypertrophy, secondary to the presence of ectopic endometrial glands within the myometrium. When the entire JZ is thickened, diffuse adenomyosis is likely ( Fig. 6 ); if only a portion of the JZ is thickened, focal adenomyosis is considered. The most widely accepted diagnostic criterion in establishing the diagnosis of adenomyosis is a JZ thickness of greater than 12 mm. This criterion reveals a diagnostic accuracy of 85%, specificity of 96%, and sensitivity of 63%. The relatively low sensitivity may relate to reduced T2 signal contrast between the JZ and dehydrated outer myometrium in some patients. Focal JZ thickening greater than 12 mm may also indicate adenomyosis but should be distinguished from transient uterine contraction.
Fig. 6
Direct and indirect features of adenomyosis. Sagittal T2WI demonstrates diffuse thickening of JZ (indirect sign), with superficial cysts in submucosal myometrium (direct sign, arrows ).
- 2.
The JZ differential was described by Dueholm and colleagues in 2001 as the difference between maximal and minimal thicknesses in the anterior and posterior uterine JZ. The investigators described a differential of greater than 5 mm as a more reliable measure than a JZ thickness of greater than 12 mm.
Pearls, Pitfalls, and Variants
Problem-solving pearls
The following problem-solving techniques may be used in challenging cases:
- 1.
Diffusion-weighted imaging is useful for distinguishing between endometrial carcinoma and focal adenomyosis. Adenomyoma demonstrates high signal on both T2WI and apparent diffusion coefficient in contrast to cellular leiomyomas and carcinoma.
- 2.
Cine MR imaging may aid in differentiating between transient myometrial contraction and focal adenomyosis.
- 3.
Susceptibility-weighted imaging is useful for detection of hemosiderin deposits in adenomyosis ( Fig. 7 ), particularly when leiomyoma is in the differential diagnosis.
Fig. 7
Susceptibility due to hemosiderin deposition. ( A ) Axial T2WI shows diffuse JZ thickening with submucosal cyst ( arrow ). ( B ) Corresponding axial, fat-saturated gradient echo T1WI demonstrates punctate foci of susceptibility artifact ( arrows ) corresponding to submucosal cyst, indicating presence of hemosiderin.
Pitfalls and Variants
Pseudolesions
- 1.
Pseudothickening of the JZ : The JZ may appear diffusely thickened during the early menstrual phase and may mimic the appearance of diffuse adenomyosis. Ideally, MR imaging should be performed midcycle to avoid this pitfall.
- 2.
Myometrial contractions : Focal bulging of the myometrium secondary to a uterine contraction creates poorly defined margins with the JZ and reduces the water content of the myometrium, which can mimic focal adenomyosis. Cine magnetic resonance (MR) and multiphase single-shot fast spin echo techniques may reveal the transient nature of these contractions.
Atypical lesions
- 1.
Adenomyomatous polyp is a rare subtype of endometrial polyp, which originates from the basal layer of the endometrium. The term “adenomatous” refers to the presence of smooth muscle components within the lesion. Histologically, this lesion may be confused with an adenomyoma. MRI features are indistinguishable from routine endometrial polyps; on T2WI, the lesion typically appears as a hypointense polypoid mass, with foci on high T2 signal.
- 2.
Adenomyotic cyst, also referred to as hemorrhagic cystic adenomyosis, is a rare subtype of adenomyosis. This lesion develops following spontaneous hemorrhage of ectopic endometrial glands, with pooling of blood products. The hemorrhage is contained by a partial or complete rim of myometrial tissue, resulting in a cyst-like appearance. The cyst may be variable in size, and may be submucosal, intramyometrial, or subserosal. Patients frequently present with dysmenorrhea and menorrhagia. Hemorrhagic contents may be high signal on T1WI, with a low signal rim on T2WI ( Fig. 8 ).
Fig. 8
Adenomyotic cyst. ( A ) Sagittal and ( B ) axial oblique T2WI reveal diffuse thickening of JZ, with focal, high-signal cyst in posterior right myometrium ( arrow ). Corresponding axial oblique T1WI with fat saturation ( C ) demonstrates T1 shortening within cyst ( arrow ), indicating hemorrhagic composition.
What Referring Physicians Need to Know: Medical Versus Surgical Management
Accurate diagnosis of adenomyosis is important in order to provide the clinician with a road map of appropriate therapeutic options. Treatment measures frequently depend on patient demographics and severity of symptoms. Hysterectomy is curative; but uterus-sparing therapies offer alternative, nonsurgical options for symptomatic patients ( Table 2 ). It is important to provide the clinician with the extent of disease involvement (focal vs diffuse), location of disease, and morphologic characteristics (cystic vs solid).
| Medical Therapies & Mechanism of Action | Surgical and Endovascular Therapies |
|---|---|
|
|
|
|
| |
|
|
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree