Müllerian duct anomalies, also called congenital uterine anomalies, are developmental structural disorders of the female genital tract. These anomalies are clinically relevant in patients with a history of infertility and pregnancy-related complications. The American Society for Reproductive Medicine classification system is the most well known, although newer systems, such as from the European Society of Human Reproduction and Embryology/European Society for Gynaecological Endoscopy, are becoming more widely accepted. MR imaging remains the optimal imaging modality due to its superior multiplanar capability and spatial resolution. This review article describes the typical MR appearance of congenital uterine anomalies.
Key points
- •
Müllerian duct developmental anomalies are clinically relevant in patients with a history of infertility and pregnancy-related complications.
- •
Müllerian duct developmental anomalies are readily characterized on MR imaging.
- •
Understanding the various classification systems for müllerian duct developmental anomalies is critical in providing accurate descriptions of the spectrum of anomalies and directing management.
Introduction
This article reviews the classification of müllerian duct anomalies (MDAs), the clinical impact of these diagnoses as related to fertility and pregnancy loss, and the MR imaging appearance of the spectrum of these congenital anomalies. MDAs are a group of congenital uterine disorders resulting from abnormalities in the embryologic development of the müllerian ducts. The prevalence of MDAs in the general population ranges between 4% and 7%. In a population of women with recurrent pregnancy loss, however, the incidence increases to as much as 18%. Most women with MDAs have little difficulty in conceiving. MDAs are associated with a higher rate of reproductive complications, however, including spontaneous abortion, intrauterine growth restriction, abnormal fetal lie, and preterm labor and birth. MDAs are commonly associated with other congenital anomalies of the urinary tract.
Embryology
During early embryonic development, the male and female genitals are indistinguishable, with 2 sets of paired ducts that are present by 6 weeks: the mesonephric (wolffian) and paramesonephric (müllerian) ducts. In the absence of müllerian inhibiting factor from male primitive gonads, the mesonephric ducts regress and the paramesonephric ducts begin to develop along the lateral aspect of the primitive kidneys. The müllerian ducts begin to develop in a bidirectional fashion, are open to the coelomic or peritoneal cavity superiorly, and are closed inferiorly.
During the 8th to 12th weeks of embryologic development, the müllerian ducts extend caudally into the primitive pelvic region where they eventually meet the urogenital sinus at the müllerian tubercle. The inferior aspect of the müllerian ducts develop into the uterus, cervix, and upper two-thirds of the vagina. The superior aspect of the müllerian ducts develops into the fallopian tubes.
By weeks 12 to 16, the septum that separates the inferior müllerian ducts begins to regress, resulting in fusion of the ducts to form a primitive uterus. Failure in septal regression results in a spectrum of MDAs. Fusion of the inferior müllerian ducts was traditionally thought to occur in a caudal to cranial direction. During formation of the uterovaginal canal, the sinus tubercle thickens and forms the sinovaginal bulbs, which create the lower 20% of the vagina. The uterovaginal canal remains separated from the sinovaginal bulbs by the horizontal vaginal plate. The vaginal plate interface with the urogenital sinus at the third month to fifth month forms the hymen.
This description, however, does not account for certain variant MDAs, such as a vertically oriented vaginal septum with an otherwise unremarkable uterus. Newer theories of septal regression that entail simultaneous bidirectional fusion in both cranial and caudal directions help explain the spectrum of anomalies.
Introduction
This article reviews the classification of müllerian duct anomalies (MDAs), the clinical impact of these diagnoses as related to fertility and pregnancy loss, and the MR imaging appearance of the spectrum of these congenital anomalies. MDAs are a group of congenital uterine disorders resulting from abnormalities in the embryologic development of the müllerian ducts. The prevalence of MDAs in the general population ranges between 4% and 7%. In a population of women with recurrent pregnancy loss, however, the incidence increases to as much as 18%. Most women with MDAs have little difficulty in conceiving. MDAs are associated with a higher rate of reproductive complications, however, including spontaneous abortion, intrauterine growth restriction, abnormal fetal lie, and preterm labor and birth. MDAs are commonly associated with other congenital anomalies of the urinary tract.
Embryology
During early embryonic development, the male and female genitals are indistinguishable, with 2 sets of paired ducts that are present by 6 weeks: the mesonephric (wolffian) and paramesonephric (müllerian) ducts. In the absence of müllerian inhibiting factor from male primitive gonads, the mesonephric ducts regress and the paramesonephric ducts begin to develop along the lateral aspect of the primitive kidneys. The müllerian ducts begin to develop in a bidirectional fashion, are open to the coelomic or peritoneal cavity superiorly, and are closed inferiorly.
During the 8th to 12th weeks of embryologic development, the müllerian ducts extend caudally into the primitive pelvic region where they eventually meet the urogenital sinus at the müllerian tubercle. The inferior aspect of the müllerian ducts develop into the uterus, cervix, and upper two-thirds of the vagina. The superior aspect of the müllerian ducts develops into the fallopian tubes.
By weeks 12 to 16, the septum that separates the inferior müllerian ducts begins to regress, resulting in fusion of the ducts to form a primitive uterus. Failure in septal regression results in a spectrum of MDAs. Fusion of the inferior müllerian ducts was traditionally thought to occur in a caudal to cranial direction. During formation of the uterovaginal canal, the sinus tubercle thickens and forms the sinovaginal bulbs, which create the lower 20% of the vagina. The uterovaginal canal remains separated from the sinovaginal bulbs by the horizontal vaginal plate. The vaginal plate interface with the urogenital sinus at the third month to fifth month forms the hymen.
This description, however, does not account for certain variant MDAs, such as a vertically oriented vaginal septum with an otherwise unremarkable uterus. Newer theories of septal regression that entail simultaneous bidirectional fusion in both cranial and caudal directions help explain the spectrum of anomalies.
Imaging techniques
Over the past few decades, a variety of imaging techniques have been developed for the characterization and classification of MDAs, including hysterosalpingography (HSG), ultrasound (US), and MR imaging.
Hysterosalpingography
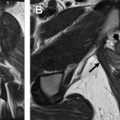
Prior to the advent of US and MR imaging, the most widely accepted imaging technique for the assessment of MDAs was HSG. The study is performed by injecting iodinated contrast material into the uterine cavity under fluoroscopic observation. A series of spot images are generally obtained as the contrast material fills the uterine cavity and passes through the fallopian tubes ( Fig. 1 ). HSG can provide reasonable evaluation of the endometrial cavity and can reliably establish tubal patency. This technique has several limitations, however. The procedure is more invasive than other imaging modalities, necessitating the insertion of a cannula into the external os or balloon-tip catheter into the endometrial cavity. More importantly, the study is of limited use in the depiction of MDAs because the myometrium and external uterine contour are not visualized. There is frequent overlap in the HSG imaging findings between different anomalies. Additionally, there is the inherent disadvantage of exposing young women to ionizing radiation. Currently, HSG plays little role in the evaluation of MDAs.
Ultrasonography
Technical advances in US have allowed for increasingly accurate evaluation of MDAs, particularly with 3-D techniques. MDAs are best evaluated during the secretory phase of the menstrual cycle when the endometrial thickness and echo complex are optimally visualized. Although conventional sagittal and transverse images of the uterus are essential, orthogonal images of the long axis of the uterus are crucial to assess the overall uterine contour. When performing US to evaluate a suspected MDA, transabdominal US should be attempted, although image acquisition may be limited by the patient’s body habitus, bowel peristalsis, bowel gas, or variation in uterine lie. Transvaginal US can provide better spatial resolution, although the field of view is inherently limited.
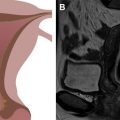
Endovaginal 3-D US allows for the creation of 3-D images from a uterine volume acquisition. After the acquisition, the data set can be manipulated to provide 3-D images of the uterus from virtually any angle. Coronal images provide essential detail regarding both the endometrial cavity and serosal surface of the uterus ( Fig. 2 ). In experienced hands, a sensitivity of 98.3% and a specificity of 99.4% have been reported in the assessment of MDAs. A high degree of concordance between 3D US and MR imaging has been shown. A recent study of surgically proved MDA cases reported a higher level of accuracy with 3-D US in comparison to MR imaging. Key advantages of this technique are lower cost and shorter examination time in experienced hands as opposed to MR imaging. Major disadvantages include the relative lack of sonographers and sonologists with adequate training in 3-D image acquisition and postprocessing techniques.
MR Imaging
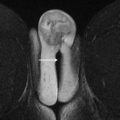
MR imaging has emerged as the universally accepted gold standard in the imaging evaluation of MDAs. Accuracies up to 100% in the evaluation of MDAs have been reported. MR imaging provides excellent delineation of both internal and external uterine anatomy. T2-weighted images are the mainstay of pelvic imaging. The endometrium is uniformly hyperintense on T2-weighted images. The junctional zone surrounds the endometrium and is readily identified as a hypointense band measuring up to 8 mm to 10 mm in thickness. The uterine myometrium surrounds the junctional zone and is of intermediate signal intensity on T2-weighted images ( Fig. 3 ). T2-weighted imaging is best performed without fat suppression due to the inherent contrast between the signal intensity of the uterus and the surrounding fat. T1-weighted images are of limited value in the evaluation of uterine zonal anatomy because the uterus is uniformly hypointense. T1-weighted images can be useful, however, for detecting hyperintense blood products in the setting of hematocolpos or endometriosis.
An inert, viscous contrast agent, such as Surgilube (HR Pharmaceuticals, Inc) or US gel, is commonly administered to distend the vagina prior to scan acquisition for a suspected MDA. Failure to adequately distend the vagina prior to imaging may preclude accurate diagnosis of complex vaginal anomalies, such as vaginal septations or vaginal duplication. Disadvantages of MR imaging are few but include high cost and potentially lengthy examination time relative to other imaging modalities. Patients with claustrophobia, excessively large body habitus, or implantable ferromagnetic medical devices may not be candidates for an MR imaging examination.
Classification systems of müllerian duct anomalies
Various classification systems have been proposed over the past several decades to describe MDAs:
- •
The American Fertility Society, now the American Society of Reproductive Medicine, (ASRM) system
- •
The vagina, cervix, uterus, adnexa, and associated malformations (VCUAM) classification
- •
The European Society of Human Reproduction and Embryology (ESHRE)/European Society for Gynaecologic Endoscopy (ESGE) classification
The most widely accepted classification system is the ASRM classification, initially proposed by Buttram and Gibbons in 1979. The system was later modified in 1988 by a subcommittee of the American Fertility Society and remains the most universally accepted system to date for classification of MDAs. This simple system is based on the degree of failure of normal development. The anomalies are divided into categories that demonstrate similar clinical manifestations and treatment ( Table 1 ).
| Classification | Description | Subcategories |
|---|---|---|
| I. Hypoplasia/agenesis | Early developmental failure of the müllerian ducts | Vaginal (I-A) Cervical (I-B) Fundal (I-C) Tubal (I-D) Combined (I-E) |
| II. Unicornuate uterus | Arrested development of one of the 2 müllerian ducts | Rudimentary horn with: Communicating uterine cavity (II-A) a Noncommunicating uterine cavity (II-B) No uterine cavity (II-C) No rudimentary horn (II-D) |
| III. Uterus didelphys | Complete failure of müllerian duct fusion | — |
| IV. Bicornuate uterus | Incomplete or partial fusion of the müllerian ducts | Complete (IV-A) b Partial (IV-B) |
| V. Septate uterus | Complete or partial failure of resorption of the uterovaginal septum | Complete (V-A) Partial (V-B) |
| VI. Arcuate uterus | Near complete resorption of the uterovaginal septum | — |
| VII. DES uterus | Related to the use of DES during the late 1940s to 1971 | — |
a Endometrial cavity that communicates with the normal side.
Although the ASRM classification remains widely accepted, the system has several drawbacks. Some particularly complex MDAs demonstrate multiple features that may encompass several ASRM categories. In a patient with disparate genital tract anomalies, it is imperative to describe each anomaly as a component part rather than attempt to classify according to the most dominant anatomic feature. Additionally, the system does not thoroughly classify vaginal anomalies. Finally, the ASRM system does not provide accurate measurements to confidently classify a uterus as bicornuate, septate, or arcuate.
The VCUAM system is designed to categorize complex malformations that defy classification by the traditional ASRM system. In the VCUAM system, each component of the genital tract is classified separately, much like the tumor-nodes-metastasis (TNM) classification for malignancies. A disadvantage of the VCUAM system is its inherent complexity, in which 56,700 individual combinations of anomalies are possible ( Table 2 ).
| Vagina | |
| 0 | Normal |
| 1a | Partial hymenal atresia |
| 1b | Complete hymenal atresia |
| 2a | Incomplete septate vagina <50% |
| 2b | Complete septate vagina |
| 3 | Stenosis of the introitus |
| 4 | Hypoplasia |
| 5a | Unilateral atresia |
| 5b | Complete atresia |
| S1 | Sinus urogenitalis (deep confluence) |
| S2 | Sinus urogenitalis (middle confluence) |
| S3 | Sinus urogenitalis (high confluence) |
| C | Cloacae |
| + | Other |
| # | Unknown |
| Cervix | |
| 0 | Normal |
| 1 | Duplex cervix |
| 2a | Unilateral atresia/aplasia |
| 2b | Bilateral atresia/aplasia |
| + | Other |
| # | Unknown |
| Uterus | |
| 0 | Normal |
| 1a | Arcuate |
| 1b | Septate <50% of the uterine cavity |
| 1c | Septate >50% of the uterine cavity |
| 2 | Bicornate |
| 3 | Hypoplastic uterus |
| 4a | Unilaterally rudimentary or aplastic |
| 4b | Bilaterally rudimentary or aplastic |
| + | Other |
| # | Unknown |
| Adnexa | |
| 0 | Normal |
| 1a | Unilateral tubal malformation, ovaries normal |
| 1b | Bilateral tubal malformation, ovaries normal |
| 2a | Unilateral hypoplasia/gonadal streak (including tubal malformation if appropriate) |
| 2b | Bilateral hypoplasia/gonadal streak (including tubal malformation if appropriate) |
| 3a | Unilateral aplasia |
| 3b | Bilateral aplasia |
| + | Other |
| # | Unknown |
| Associated Malformation | |
| 0 | None |
| R | Renal system |
| S | Skeleton |
| C | Cardiac |
| N | Neurologic |
| + | Other |
| # | Unknown |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree