Endometrial cancer is the most common gynecologic malignancy in the United States, with recent increasing incidence mostly owing to obesity. Preoperative MR imaging is essential to stratify patients according to their risk of recurrence and to guide surgical management. In the combination of T2-weighted imaging, diffusion-weighted imaging, and dynamic contrast enhancement, MR imaging provides a “one-stop shop” approach for patient-specific accurate staging including the evaluation of the depth of myometrial invasion, cervical stromal invasion, extrauterine extension, and lymph node status.
Key points
- •
Endometrial cancer incidence is increasing owing mostly to Western lifestyle.
- •
Preoperative MR imaging helps in the selection of patients who may benefit from minimally invasive surgery.
- •
The combination of T2-weighted, diffusion-weighted, and dynamic contrast-enhanced MR imaging provides a “one-stop shop” approach for the accurate staging of patients with endometrial cancer.
- •
Sequences angled perpendicularly to the endometrial cavity are critical to accurately assess the depth of myometrial invasion.
Introduction
Endometrial cancer is the fourth most common malignancy in women, with more than 60,000 newly diagnosed cases in the United States in 2016. Its incidence is increasing, mainly owing to increased life expectancy and obesity rates. Approximately 75% of cases occur in postmenopausal women, with a mean age at presentation of 63 years. Most endometrial cancers are diagnosed at an early stage (80% stage I), with 5-year survival rates of more than 95%.
Endometrial cancer is staged surgically using the International Federation of Gynecology and Obstetrics (FIGO) system. The standard surgical staging procedure consists of hysterectomy, bilateral salpingo-oophorectomy, lymph node dissection, peritoneal washing, and omental biopsies. However, although the FIGO stage correlates with prognosis, preoperative staging is essential to tailor treatment. Indeed, early stage patients may be treated appropriately with minimally invasive surgery and without lymphadenectomy. This approach leads to reduced morbidity and shorter duration of hospital stay, with an outcome comparable to the standard, more extensive staging procedure. The effective implementation of this management approach relies on accurate preoperative staging.
MR imaging is the imaging modality of choice to determine the depth of myometrial invasion preoperatively, which in turn correlates with tumor grade, presence of lymph node metastases, and overall survival. MR imaging is recommended by the American College of Radiologists and the European Society of Radiology for preoperative endometrial cancer staging. The combination of T2-weighted imaging (T2WI), dynamic contrast-enhanced (DCE) MR imaging, and diffusion-weighted imaging (DWI) offers the best diagnostic accuracy in staging of endometrial cancer.
In this review, we emphasize the advantages and challenges of MR imaging staging of endometrial cancer, especially focusing on the MR imaging acquisition protocol and the role of DWI and DCE MR imaging.
Introduction
Endometrial cancer is the fourth most common malignancy in women, with more than 60,000 newly diagnosed cases in the United States in 2016. Its incidence is increasing, mainly owing to increased life expectancy and obesity rates. Approximately 75% of cases occur in postmenopausal women, with a mean age at presentation of 63 years. Most endometrial cancers are diagnosed at an early stage (80% stage I), with 5-year survival rates of more than 95%.
Endometrial cancer is staged surgically using the International Federation of Gynecology and Obstetrics (FIGO) system. The standard surgical staging procedure consists of hysterectomy, bilateral salpingo-oophorectomy, lymph node dissection, peritoneal washing, and omental biopsies. However, although the FIGO stage correlates with prognosis, preoperative staging is essential to tailor treatment. Indeed, early stage patients may be treated appropriately with minimally invasive surgery and without lymphadenectomy. This approach leads to reduced morbidity and shorter duration of hospital stay, with an outcome comparable to the standard, more extensive staging procedure. The effective implementation of this management approach relies on accurate preoperative staging.
MR imaging is the imaging modality of choice to determine the depth of myometrial invasion preoperatively, which in turn correlates with tumor grade, presence of lymph node metastases, and overall survival. MR imaging is recommended by the American College of Radiologists and the European Society of Radiology for preoperative endometrial cancer staging. The combination of T2-weighted imaging (T2WI), dynamic contrast-enhanced (DCE) MR imaging, and diffusion-weighted imaging (DWI) offers the best diagnostic accuracy in staging of endometrial cancer.
In this review, we emphasize the advantages and challenges of MR imaging staging of endometrial cancer, especially focusing on the MR imaging acquisition protocol and the role of DWI and DCE MR imaging.
Patient population and tumor type
Incidence and Risk Factors
Endometrial cancer is the most common gynecologic cancer in North America ( Box 1 ). Furthermore, the number of cases is projected to increase by 55% in the United States between 2010 and 2030. The increasing incidence is thought to be mainly related to the Western lifestyle and obesity in particular. Obesity increases estrogen production via its aromatization in adipose tissues. Diabetes mellitus is associated with an increased risk, probably related to concurrent obesity, although an independent association between diabetes and endometrial cancer has been reported. Nulliparity and infertility are additional risk factors, including polycystic ovarian syndrome. Other risk factors for endometrial cancer include unopposed estrogen therapy such as tamoxifen, estrogen-producing tumors, and early menarche/late menopause. Most cases of endometrial cancer are sporadic; however, 5% have an hereditary basis related to the Lynch syndrome. This syndrome is due to germ-line mutations of one of the DNA repair genes MSH2, MLH1, and MSH6.
Excess estrogen exposure
Exogenous estrogen or estrogen agonists
- •
Unopposed estrogen therapy
- •
Tamoxifen
- •
Estrogen–progestin postmenopausal hormone therapy
- •
Phytoestrogens
- •
Endogenous estrogen
- •
Obesity
- •
Chronic anovulation
- •
Early menarche and late menopause
- •
Estrogen secreting tumors
- •
Age
Family history of Lynch syndrome
Associated factors
Nulliparity and infertility
Diabetes
Breast cancer
Tubal ligation
Protective factors
Hormonal contraceptives
Increased maternal age
Smoking
Diet and exercise
- •
Physical activity
- •
Tea
- •
Coffee
- •
Pathogenesis
Endometrial carcinomas have been traditionally divided into 2 subtypes based on prognosis ( Table 1 ). Type 1 endometrial carcinomas are estrogen-dependent tumors and include FIGO grades 1 and 2 endometrioid adenocarcinomas. Type 2 endometrial carcinomas include serous papillary, clear cell adenocarcinomas, carcinosarcomas, and FIGO grade 3 endometrioid adenocarcinomas. Type II tumors are not driven by estrogen and tend to present at a higher stage and behave more aggressively. Several genomic and molecular characteristics support this dichotomous classification and have become an integral component of the pathologic evaluation. Type I tumors are associated preferentially with genetic alterations in PTEN, KRAS, CTNNB1, and PIK3CA, whereas serous carcinomas usually harbor TP53 mutations. The Cancer Genome Atlas Research Network has improved the evaluation of the molecular landscape of endometrial cancer significantly by describing 4 molecular subtypes, including POLE, the smallest group with an excellent prognosis ; microsatellite unstable tumors ; copy number low microsatellite stable tumors and ; copy number high tumors with mostly TP53 mutations. This latter group includes serous carcinomas and is associated with a poor prognosis.
| Type I Endometrial Carcinoma | Type II Endometrial Carcinoma | |
|---|---|---|
| Hormone dependency | Estrogen dependent | Non estrogen dependent |
| Percentage | 80–85 | 10–15 |
| Age at diagnosis | Younger patient (premenopausal to perimenopausal) | Older (postmenopausal) |
| Risk factors | Obesity Unopposed estrogen exposure Nulliparity and infertility (polycystic ovary syndrome) | None |
| Histologic features | Low-grade tumor Endometrioid grades 1–2 Endometrioid with squamous differentiation Mucinous | High-grade tumor Endometrioid grade 3 Clear cell Serous Undifferentiated Carcinosarcoma |
| Precursor | Arising from endometrial hyperplasia | Endometrial intraepithelial carcinoma |
| Associated genetic factor | PTEN or KRAS gene mutation Microsatellite instability | P53 mutation |
| Clinical course | Early initial stage (70%) Slow growing Local recurrence | Advanced initial stage (60%) Rapid progression Aggressive behavior with poor prognosis Abdominal and lymphatic recurrence |
| 5-year overall survival | 80% | 40% |
Diagnosis
Abnormal vaginal bleeding is the most common initial presentation of endometrial cancer. The standard diagnostic evaluation includes pelvic ultrasound and pipelle endometrial biopsy or dilatation and curettage. Thresholds of endometrial thickness of 4 or 5 mm have been proposed to detect endometrial cancer with a sensitivity of up to 95% and specificity of 77% in postmenopausal women. A recent metaanalysis suggested a more stringent threshold of 3 mm to achieve a higher sensitivity (97.5%).
MR imaging protocol
Patient Preparation
- •
To diminish artifacts owing to peristalsis, patients are typically instructed to fast for 4 to 6 hours before MR imaging. An antiperistaltic agent, such as butylscopolamine bromide or glucagon, is usually administered intramuscularly or intravenously at the beginning of the examination.
- •
Patients are also instructed to empty their urinary bladder to decrease phase ghost artifacts related to bladder motion and filling.
- •
Imaging is performed with the patient in the supine position using a multichannel phase array surface coil. Saturation bands placed along the subcutaneous fat of the anterior and posterior body wall are useful to diminish near-field artifact.
- •
Endometrial cancer is often diagnosed after dilation and curettage. MR imaging interpretation is usually not affected adversely by uterus changes after this procedure and MR imaging can be performed after vaginal bleeding has stopped.
MR Imaging Protocol
The combination of conventional (T2WI) and functional sequences (DWI and DCE MR imaging) provides a “one-stop shop” approach for the staging of patients with endometrial cancer ( Table 2 ).
| Coronal T2 | Axial T1 | Axial T2 | Sagittal T2 | Axial Oblique T2 | Sagittal DWI | Axial Oblique DWI | Sagittal DCE | Axial Oblique DCE | |
|---|---|---|---|---|---|---|---|---|---|
| FOV (cm) | 36–44 | 32–36 | 20–24 | 20–24 | 20–24 | 20–24 | 20–24 | 20–24 | 20–24 |
| Thickness (mm) | 6 | 5 | 4 | 4 | 4 | 4 | 4 | 4 /−2 | 4 /−2 |
| Gap (mm) | 1 | 1 | 0.4 | 0.4 | 0.3 | 0 | 0 | 0 | 0 |
| Echo time (ms) | 104 | Min | 120 | 120 | 120 | Min | Min | 2.1 | 2.1 |
| Relaxation time (ms) | 500–1600 | Min | 3500–4000 | 3500–4000 | 4000–4500 | 5000 | 5000 | 6.4 | 6.4 |
| Flip angle | 90 | 90 | 90 | 90 | 90 | N/A | N/A | 80 | 80 |
| Bandwidth (kHz) | 32 | 32 | 32 | 32 | 32 | N/A | N/A | 83 | 83 |
| ETL | 200 | 3–4 | 28 | 28 | 28 | N/A | N/A | N/A | N/A |
| NEX | 1 | 2 | 3 | 3 | 3 | 6 | 6 | 1 | 1 |
| Frequency steps | 300 | 448 | 384 | 384 | 384 | 90 | 90 | 320 | 320 |
| Phase encoding steps | 200 | 224 | 256 | 256 | 256 | 192 | 192 | 280 | 280 |
Conventional sequences
The conventional protocol includes small field of view (FOV) high-resolution axial, and sagittal, T2WI within the pelvis and large FOV axial T1-weighted imaging or T2WI of the abdomen and pelvis. A large FOV coronal T2 on the whole abdomen and pelvis may be added optionally.
In addition, a high-resolution, small FOV, axial oblique T2WI angled perpendicularly to the endometrial cavity is critical to assess the depth of myometrial invasion.
Image acquisition optimization for T2WI angled perpendicularly to the endometrium
- •
Thin section (3–4 mm).
- •
Small FOV (20–24 cm).
- •
The uterus can have a variable position within the pelvis and it may be tilted to the left or right of the midline. If oblique axial images are prescribed using only sagittal images, this may not yield an imaging plane that is precisely perpendicular to the endometrial cavity. A “double oblique images” angled both in the sagittal and coronal planes create a “ true oblique” that is exactly orthogonal to the endometrial cavity ( Figs. 1 and 2 ).
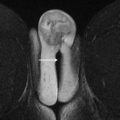
Fig. 1
The “double oblique T2”: Drawing showing a 3-dimensional anteverted and right-sided uterus. To get a true axial acquisition to uterine body, a double axial oblique sequence perpendicular to both the sagittal and coronal axis of the uterus must be acquired. A, anterior; L, left; P, posterior; R, right.
Fig. 2
Sagittal ( A ) and coronal ( B ) T2-weighted imaging sequences showing an anteverted ( A ) and right tilted ( B ) uterus. Angling the axis along the sagittal and coronal orientation of the uterus enables a true double oblique sequence ( C ) with an accurate evaluation of the depth of myometrial invasion.
Functional MR imaging
Diffusion-weighted imaging
An imaging protocol should also include DWI in at least 1 but preferably in 2 planes (axial oblique along the uterus with the same orientation as axial oblique T2WI and sagittal) with a minimum of 2 b values (eg, b = 400, b = 800). Acquiring T2WI and DWI on the same plane allows images fusion and optimizes anatomic correlation.
DWI is traditionally implemented as an echoplanar imaging sequence. However, echoplanar imaging is highly prone to susceptibility artifacts and image blurring owing to the relatively long gradient echo train used for image acquisition. Parallel imaging is now widely used to reduce the echo train length and, thus, geometric distortions inherent to echoplanar imaging. Recently, FOCUS imaging (FOV optimized and constrained undistorted single-shot DWI) has been investigated in prostate and endometrial cancer. This new acquisition technique uses a 2-dimensional, spatially selective echoplanar radiofrequency excitation pulse and a 180° refocusing pulse reducing the FOV in the phase-encoding direction. Reduced phase direction FOV technique improves the spatial resolution, without associated phase wrapround artifact and with decreased artifacts related to motion and susceptibility, which are common with a large FOV ( Fig. 3 ).
Dynamic Multiphase Contrast-Enhanced MR Imaging
DCE MR images are obtained with a 3-dimensional gradient echo T1WI, fat-saturated sequence after the administration of 0.1 mmol/kg of gadolinium at a rate of 2 mL/s. The most commonly used sequences are LAVA (GE, Fairfield, CT), VIBE (Siemens, Munich, Germany), and THRIVE (Philips, Amsterdam, the Netherlands). Images are traditionally acquired before contrast injection and then during multiple phases of enhancement in sagittal planes at 25 seconds, 1 minutes, and 2 minutes after injection; a delayed sequence is acquired on axial oblique 4 minutes after injection.
- •
Early phase images (25 seconds to 1 minute after contrast administration) are optimal for the detection of subendometrial enhancement that corresponds with the inner junctional zone, which enhances earlier than the rest of the myometrium. Uninterrupted subendometrial linear enhancement nearly excludes superficial myometrium invasion.
- •
Equilibrium phase images (2–3 minutes after injection) are best for the evaluation of deep myometrium invasion.
- •
Recently, an imaging delay of approximately 90 seconds has been reported as the optimal timing delay for best tumor–myometrium contrast.
- •
Delayed phase images (4–5 minutes after injection) are optimal for the detection of cervical stroma invasion.
Dynamic contrast-enhanced MR imaging cancer detection and diagnosis
Endometrial cancer is typically diagnosed by endometrial sampling. However, in some cases endometrial sampling is not possible, owing to cervical stenosis for instance, or its results are inconclusive. In these situations, MR imaging is helpful in cancer depiction.
Classic Tumor Appearance on MR Imaging
Three distinct zones are apparent on T2WI of the uterus: (1) the high signal intensity of endometrium surrounded by (2) the low signal intensity junctional zone (inner myometrium), which in turn is surrounded by (3) the intermediate signal intensity outer myometrium.
- •
On T2WI, the tumor usually demonstrates intermediate to low T2 signal intensity relative to the hyperintense normal endometrium ( Fig. 4 ). However, small tumors may not be associated with endometrial thickening or can have a signal intensity similar to that of the normal endometrium. In those cases, functional sequences may be particularly helpful.
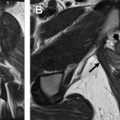
Fig. 4
Stage IA endometrial cancer. ( A ) Sagittal and ( B ) axial oblique T2-weighted imaging (T2WI) shows relative small distention of the endometrial cavity by an intermediate signal intensity tumor ( arrow ) within the fundus. ( C ) Fused axial obliqueT2WI–diffusion-weighted imaging (DWI) shows a small focus of hyperintense DWI signal within the endometrial cavity ( arrow ) in keeping with stage IA tumor invading less than 50% of the myometrium.
- •
On DCE MR imaging, small tumors may enhance early compared with the normal endometrium. In the later phases of enhancement, these tumors may appear hypointense relative to the myometrium.
- •
On DWI, tumors appear hyperintense on the high B-value DWI image with corresponding hypointense signal on the apparent diffusion coefficient (ADC) map (see Fig. 4 ).
Data are accumulating to support the use of DWI as a tool to differentiate endometrial cancer from normal endometrium or benign uterine disorders. Multiples studies have shown that ADC values of endometrial cancers are significantly lower than those of endometrial polyps and normal endometrium. In a cohort, of 70 cancer patients and 36 control subjects, Rechichi and colleagues found that there was no overlap in ADC values between endometrial cancer and normal endometrium. The authors were able to define using 2b values (b = 0, b = 1000) and an ADC value threshold of 1.05 × 10 −3 mm 2 /s to distinguish endometrial cancer from normal endometrial tissue. Others have proposed cutoffs ranging from 1.05 to 1.28 to distinguish malignant from benign lesions with a range of sensitivities of 60.1% to 87% and specificity of 100%. This information might be of particular relevance when endometrial biopsy is not possible or inconclusive.
Dynamic contrast-enhanced MR imaging cancer staging
Stage I
Stage IA tumors invade less than 50% of the myometrial thickness and stage IB tumors involve more than 50% of the myometrial thickness ( Tables 3 and 4 ).
| T2 |
|
| DCE MR imaging |
|
| DWI |
|
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree