Etiology
Benign pleural thickening caused by fibrosis is the second most common pleural abnormality, the most common one being effusion. Pleural fibrosis has a number of causes and is the outcome of many pleural diseases and a potential complication of every inflammatory condition that affects the lungs.
The pleura show a variety of patterns of fibrosis. These may be either localized (apical caps, pleural plaques) or diffuse. In some respects pleural fibrosis can be considered defective healing. Central to the pathogenesis of pleural fibrosis is inflammation of the pleural cavity. The response of the mesothelial cell to injury and its ability, along with the underlying basement membrane, to maintain its integrity are crucial in determining whether normal healing or pleural fibrosis ensues.
The pleura acts not only as a protective barrier but as an immunologically and metabolically responsive membrane that is involved in maintaining a dynamic homeostasis in the pleural space. The mesothelial cells secrete glycosaminoglycans and other surfactant-like molecules to lubricate the pleural surface in addition to proinflammatory, antiinflammatory, and other immunomodulatory mediators, factors that promote deposition and clearance of fibrin, and growth factors and extracellular matrix proteins to aid in serosal repair. Pleural injury and fibrosis are characterized by disordered fibrin turnover. Interactions among mesothelial and inflammatory cells, cytokines, growth factors, and blood-derived products are important in the pathogenesis of tissue fibrosis. The exact timing of these interactions, as well as genetic factors, may be the difference between resolution of an injury, leading to normal repair and regeneration, and excessive matrix formation with fibrosis and scar formation.
Most of the diffuse forms of visceral or parietal pleural fibrosis result from organization of recurrent effusions. The most common causes are collagen vascular diseases, asbestos exposure, and drugs, especially methysergide, methotrexate, bromocriptine, practolol, and mitomycin.
Pathophysiology
Anatomy
Pleuritis with associated pleural effusion develops in a number of different diseases. In most cases the pleural inflammation regresses, with or without medication and without sequelae of pleural fibrosis. However, in others, pleural inflammation progresses to pleural fibrosis. In experimental models of pleuritis, the mesothelial cells proliferate and become reactive in response to injury. Reactive mesothelial cells become columnar with increased microvilli and upregulation of the enzymes of the oxidative pathway. Fibrinolysis and synthesis of prostacyclins and hyaluronic acid–rich glycoproteins are stimulated in an attempt to remove debris and to repair the mesothelial cell surface, maintaining the integrity of the pleural surface. The degree of mesothelial cell and basement membrane injury and regeneration appears to be the key to whether complete recovery or pleural fibrosis occurs.
For pleural fibrosis to develop, an increase in the extracellular matrix, characterized by a disorder in fibrin turnover, must form to allow the fibrotic process to continue. The fibrinous matrix is created by the release of coagulation proteins from the plasma in response to pleural space inflammation. A complex balance exists between procoagulant and fibrinolytic activities of the mesothelial cells. In exudative pleuritis, local equilibrium between these activities is disrupted, enhancing fibrin deposition. Exudative pleural effusions are characterized by increased procoagulant and depressed fibrinolytic activity, favoring fibrin deposition in the pleural space.
The Apical Cap
Thickening of the pleura over the apices of the thorax and occasionally in the interlobar fissures is fairly common. In most cases the etiology is unknown. Such “idiopathic” caps usually measure less than 5 mm in height and have a smoothly marginated or undulating lower margin. Apical caps, either unilateral or bilateral, are a common feature of advancing age. A unilateral cap was seen in 11%, and bilateral caps were seen in 12% on a radiographic study. The prevalence increased with age, being identified in 6% of patients younger than 45 years and in 16% of those older than 45 years. The prevalence is similar in men and women. There is no significant association of apical caps with tuberculosis, emphysema, diffuse interstitial fibrosis, silicosis, or asbestosis.
Pathology
Apical caps consist mainly of extrapleural fat and a combination of parenchymal and to a lesser extent pleural fibrosis. On histologic examination the pleura is thickened by dense, sometimes hyalinized collagen, accompanied in some cases by small foci of mononuclear inflammatory cells. The architecture of the underlying lung parenchyma is preserved, but the airspaces are obliterated by fibrous tissue; elastic tissue in the alveolar septa is typically increased. Focal areas of calcification or ossification can be seen. The pathogenesis of the fibrosis is uncertain. In one autopsy study an association with histologic evidence of chronic bronchitis and pulmonary artery narrowing was identified. The investigators suggested that intermittent or continuing low-grade infection combined with relative apical ischemia might be responsible for the fibrosis.
Manifestations of the Disease
Radiography
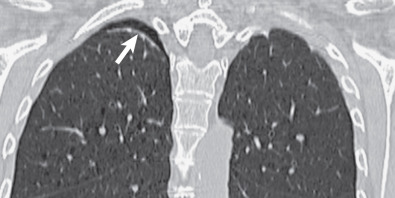
The apical cap is an irregular, nonhomogeneous opacity located at the extreme apex of the lung. The lower border is usually sharply marginated, but it is frequently tented or undulating ( Fig. 73.1 ). The cap opacity is of variable thickness (usually <5 mm in craniocaudal dimension) and of variable transverse diameter. It may be unilateral or bilateral. In patients with a cap related to prior tuberculosis, the apical opacity can measure more than 1 cm in thickness on the radiograph ( Fig. 73.2 ).
Computed Tomography
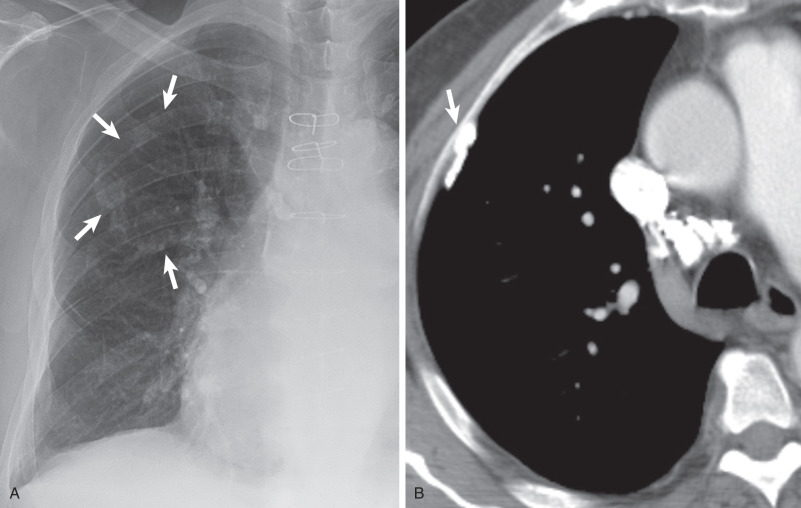
Extrapleural fat with interspersed vessels accounts for most of the opacity seen on the radiograph (see Figs. 73.1 and 73.2 ). Extrapleural fat is commonly thicker adjacent to areas of chronic pleural disease, possibly resulting from retraction of the lung from fibrosis. An apical pneumothorax can outline the apical cap ( Fig. 73.3 ). The thin white visceral line will be thickened in this case and is well depicted in the presence of air in the pleural space.
Differential Diagnosis
Other lesions arising in the lung, pleura, or extrapleural space may produce unilateral or bilateral apical caps. These include the following:
- •
Neoplasm: Pancoast (superior sulcus) tumor ( Fig. 73.4 ), lymphoma extending from the neck or mediastinum, metastases, and mesothelioma
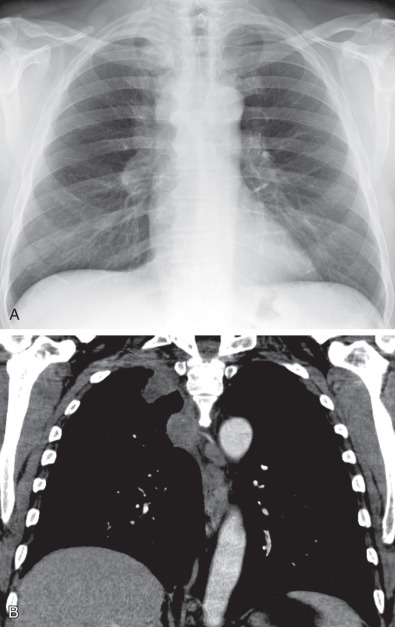
Fig. 73.4
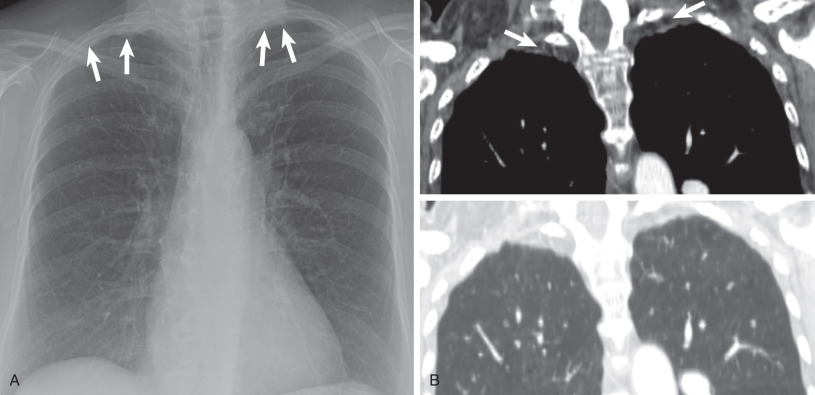
Pancoast tumor. (A) Posteroanterior chest radiograph and (B) coronal contrast-enhanced CT scan show a soft tissue mass in the right lung apex and thickening of the right paratracheal stripe representing lymph node metastases.
- •
Infection: Extrapleural abscesses or tuberculosis extending from the neck
- •
Radiation fibrosis: After treatment for lymphoma, head and neck or breast carcinoma ( Fig. 73.5 )
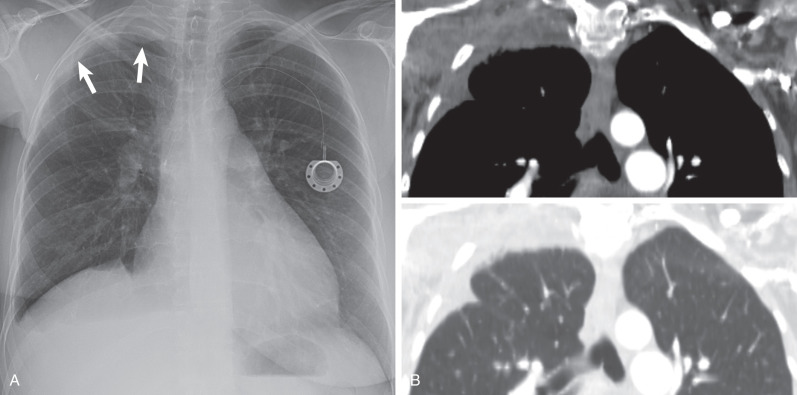
Fig. 73.5
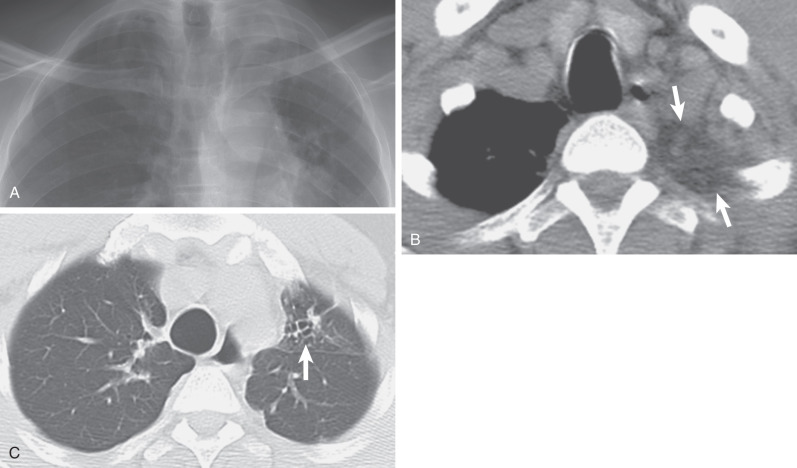
Radiation fibrosis. (A) Posteroanterior chest radiograph demonstrates asymmetric right apical pleural thickening (arrows) postradiation therapy for breast cancer. Note is made of a smaller right breast shadow. (B) Composite image with coronal reformatted CT scans in soft tissue and lung windows shows the radiographic opacity is composed of extrapleural fat, pleural thickening, and reticulations from pulmonary fibrosis. Note right upper lobe volume loss.
- •
Hemorrhage: Extrapleural dissection of blood from a ruptured aorta, fractures of the ribs or spine, or bleeding resulting from subclavian line placement
- •
Vascular anomalies: Coarctation of the aorta with dilated collaterals over the apex, fistula between the subclavian artery and vein
- •
Miscellaneous: Mediastinal lipomatosis with subcostal fat extending over the apices
- •
Pleural effusions: In a supine patient may also simulate an apical cap, as the fluid tracks to the most dependent portion of the pleural space ( Fig. 73.6 )
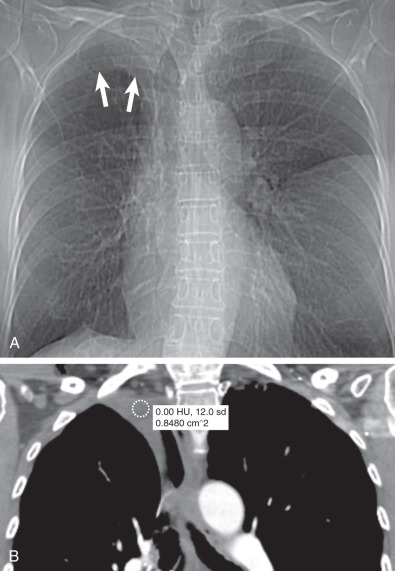
Fig. 73.6
Loculated pleural effusion post right upper lobectomy. (A) Scout view of CT demonstrates right apical opacity mimicking pleural thickening (arrows) . (B) Coronal reformatted CT image shows loculated right apical pleural fluid with density of 0 Hounsfield units.
- •
Typically bilateral and symmetric
- •
Measure <5 mm in thickness
- •
Most commonly idiopathic
- •
Prevalence increases with age
Pleural Plaques
Etiology, Prevalence, and Epidemiology
Pleural plaques are the most common manifestation of asbestos inhalation (see Chapter 61 ). Asbestos-induced fibrosis can involve the pulmonary interstitium (asbestosis), the parietal pleura (pleural plaques), or the visceral and parietal pleura (diffuse pleural thickening). The pleura appears to be more sensitive than the lung parenchyma to the effects of asbestos fibers. Pleural plaques occur with lower inhaled fiber burdens, whereas asbestosis is associated with higher fiber burdens. Pleural plaques serve as a marker of exposure and may be seen with brief or slight exposure. Although the overall incidence of plaques increases with dose, a linear correlation does not exist between plaque severity and total dust exposure. However, a direct relationship has been found between the intensity of asbestos exposure and total area of pleura involved by the plaques in a postmortem study. Among environmentally exposed individuals, the prevalence ranges from 0.53% to 8%. Studies of occupationally exposed individuals show frequencies of 3% to 14% in dockyard workers and up to 58% among insulation workers. However, the previously reported prevalence figures must be considered in the context of the method used to detect pleural plaques.
There is a latency period of 20 to 30 years between asbestos exposure and the development of pleural plaques. Calcification is seen in 15% of patients after a latency period of 30 to 40 years, with calcification of plaques increasing with time from exposure. Diaphragmatic calcification is highly suggestive of previous exposure to asbestos.
There are other less common causes of pleural plaques besides asbestos. Silicosis has been described to cause pleural plaques; however, an autopsy study demonstrated that both visceral pleura and parietal pleura are involved, whereas asbestos plaques typically involve the parietal pleura. Chest trauma (hemothorax), infection (empyema), and artificial pneumothoraces previously used for the treatment of tuberculosis can also cause pleural plaques. In contrast to asbestos-induced pleural plaques, these lesions are usually unilateral and may be upper zonal in distribution.
Clinical Presentation
Plaques are frequently diagnosed incidentally on chest radiographs or computed tomography (CT) scans and have important medicolegal significance. Patients with pleural plaques are generally not symptomatic in the absence of associated pulmonary disease or diffuse pleural fibrosis.
Pathophysiology
Whereas microscopic fragments of asbestos fibers have been isolated from pleural plaques, it remains unclear how fibers reach the parietal pleura and if they are responsible for inflammation and fibrosis. Chrysotile fibers, fine, short, and especially fragmented fibers, are better able to reach the pleura and probably account for many of the pathogenetic and anatomic features of asbestos-related disease. Some have proposed that pleural plaques are the direct result of local inflammation of the parietal pleura from asbestos fibers that protrude out of the visceral pleural surface. However, there have been no confirmative pathologic data to support this. The most plausible explanation for plaque pathogenesis is that the asbestos fibers reach the parietal pleura by retrograde lymphatic drainage involving flow from the mediastinal lymph nodes to the retrosternal and intercostal lymphatics. Asbestos fibers may also embolize to the parietal pleura through the costal vascular supply. Plaques are located in the vicinity of stomas in the parietal pleura, so-called Kampmeier foci, where asbestos fibers are resorbed by lymphatic flow.
These stomas are typically located in the lower hemithorax, which is why most plaques tend to occur below the sixth ribs.
Pathology
Pleural plaques are discrete elevated areas of hyaline fibrosis almost invariably arising from the parietal pleura. They consist of acellular bundles of collagen in an undulating basket-weave pattern and may contain abundant numbers of asbestos fibers, almost exclusively chrysotile fibers, but asbestos bodies (ferruginous bodies containing an asbestos core) are absent. The inner side is covered by normal mesothelial cells; on the costal side there may be signs of low-grade inflammation.
Pleural plaques may progress over time, in the absence of continued exposure. Typically, they will increase the amount of calcification over time. There is no evidence that pleural plaques undergo malignant degeneration into mesothelioma.
Pleural plaques are discrete, elevated, opaque, shiny, rounded lesions ( Fig. 73.7 ). Thin plaques are smooth and gray-white. Thick plaques are ivory colored or gray and may have a smooth or nodular surface. On microscopic examination plaques are seen to be laminated collagenous connective tissue, acellular, with few inflammatory or fibrocystic nuclei ( Fig. 73.8 ); many are covered with a thin layer of regular or well-differentiated mesothelial cells. Elastic staining shows intact lamellae beneath the plaque in continuity with the surrounding normal parietal pleural connective tissue. This suggests that plaques are extrapleural and develop between the pleural connective tissue and the covering layer of mesothelial cells.
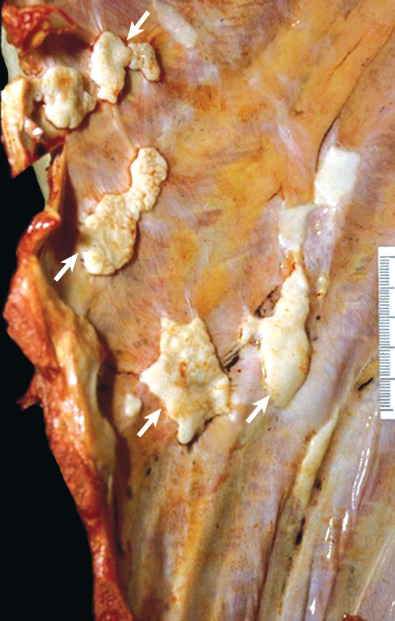
Parietal pleural plaques are well-demarcated lesions that most commonly occur between the sixth through ninth ribs in the posterolateral thorax. They occur more frequently in the lower hemithorax. The costophrenic angles and apices are spared. Plaques may be found over the dome of the diaphragm and on the pericardium.
Lung Function
Pleural plaques are not usually associated with impairment of lung function. As pleural plaques are covered by a normal mesothelium and are not associated with adherence between the lung and the chest wall during respiratory movements, the lung can still slide along the chest wall and can fully expand. It has been suggested that when there is an impairment of lung function, it is more likely due to subclinical interstitial fibrosis.
Manifestations of the Disease
Radiography
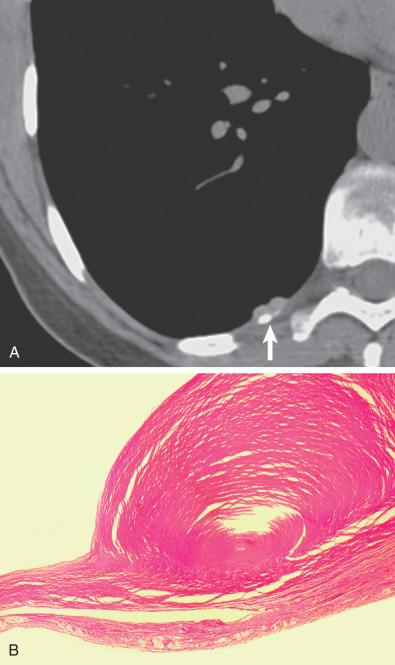
The earliest manifestation of a pleural plaque on the chest radiograph is as a unilateral or bilateral thin line of soft tissue density under a rib in the axillary region, usually the seventh or eighth rib. Noncalcified pleural plaques are difficult to identify on the chest radiograph except when the x-ray beam is tangential to the plaque. The plaque appears in profile as a sharply marginated dense band of soft tissue ranging from 1 to 10 mm in thickness, paralleling the inner margin of the lateral thoracic wall. As with any extraparenchymal lesion, pleural plaques viewed en face can be difficult to see. They have a characteristically sharply defined medial edge and an ill-defined lateral margin, the so-called incomplete border sign ( Fig. 73.9 ). The holly leaf sign refers to the radiographic appearance of pleural plaques with irregular thickened nodular edges ( Fig. 73.10 ). The majority of asbestos-related plaques occur in the parietal pleura. Visceral pleural plaque formation is uncommon. The radiographic diagnosis of visceral pleural thickening is not reliable unless it occurs in the interlobar fissures ( Fig. 73.11 ). Recognition of a calcified pleural plaque in a thickened minor fissure or major fissure has been reported. These visceral plaques usually are associated with extensive parietal pleural disease.
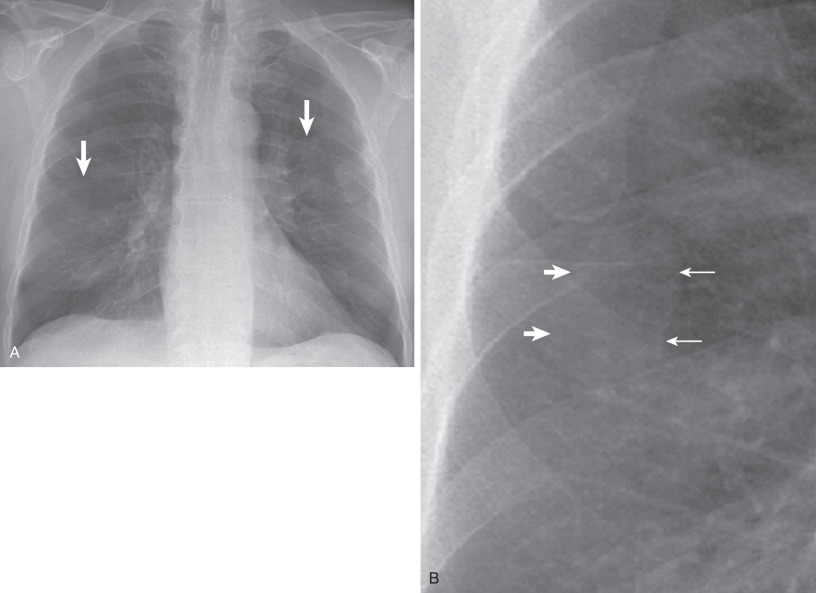
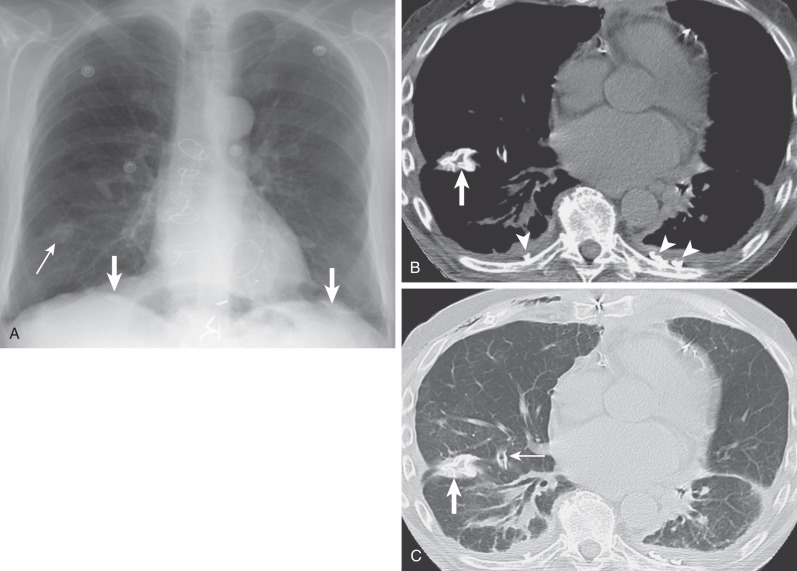
The chest radiograph has reported sensitivities of 30% to 80% in the detection of pleural plaques. Detection is dependent on a number of factors, including plaque thickness, size, and position; radiographic technical factors; and the presence of calcification.
The International Labour Office (ILO) classification of pneumoconioses assesses asbestos-induced pleural disease on chest radiography using the posteroanterior projection. Autopsy studies report a high false-negative rate for the radiographic detection of pleural plaques.
Computed Tomography
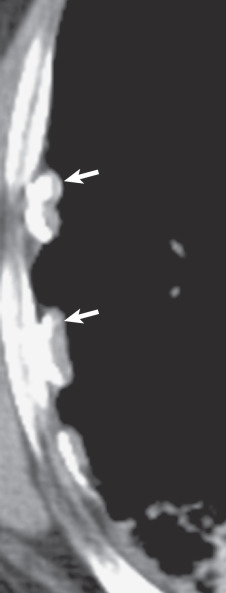
On CT a pleural plaque is defined as a discontinuous soft tissue focal thickening of the pleural surface, with or without foci of calcification. Plaques typically have edges that are thicker than the central portion ( Fig. 73.12 ; see also Fig. 73.8 ) and can progress in size, extent, and calcification with time. Pleural plaques are internal to and separated from the ribs by a thin fat layer ( Fig. 73.13 ). This fat layer also separates the thickened pleura from the innermost intercostal muscle, subcostal muscle, and intercostal vein. Intercostal vessels may cause normal extrapleural soft tissues to appear thicker in the paravertebral regions. Paraspinal pleural plaques should be diagnosed only if thickening is visible at multiple levels, extrapleural fat is visible between the pleura and intercostal vessels, thickened pleura is seen to indent the adjacent lung, or there is dystrophic calcification within the plaque.
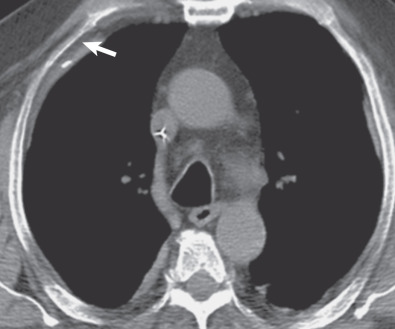
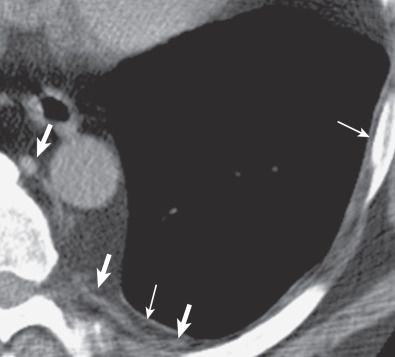
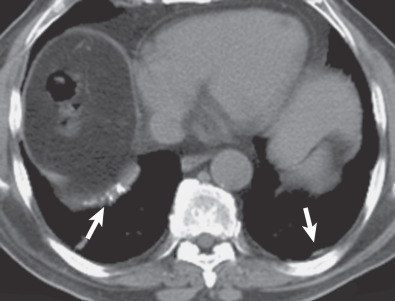
The characteristic sites for pleural plaques related to asbestos exposure are the posterolateral chest wall between the sixth and ninth ribs, the dome of the diaphragm ( Fig. 73.14 ), and the mediastinal pleura, particularly over or involving the pericardium ( Fig. 73.15 ). Typically, plaques are not present at the apices or the costophrenic angles. Plaques that arise from the visceral pleura are rare and usually found in the lower aspects of the interlobar fissures; they may calcify and are usually associated with extensive parietal pleural plaque formation. The size and number of pleural plaques are variable. Pleural plaques are bilateral in the majority of asbestos-exposed individuals, but they can also be unilateral. Unilateral plaques can also be related to prior hemothorax ( Fig. 73.16 ) or empyema (see Fig. 73.10 ). According to the literature, calcification of plaques is seen in 10% to 15% of cases, although many radiologists find that percentage to be much lower than expected based on their clinical practice where the majority of pleural plaques are calcified.
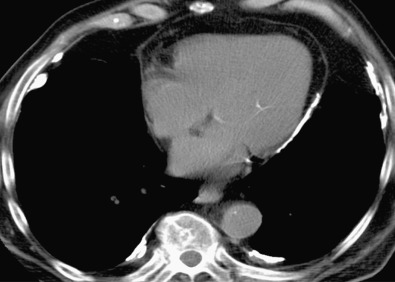
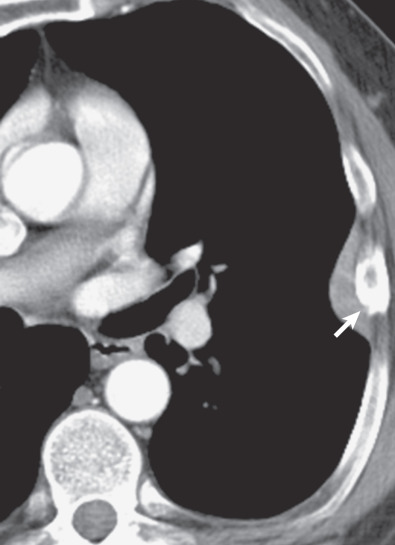
Some plaques demonstrate subtle reticular densities in the adjacent lung, giving rise to the term hairy plaques. This usually represents compressive atelectasis in asymptomatic patients or potentially subclinical fibrosis in some patients who demonstrate functional impairment but who do not manifest any other evidence of pulmonary asbestosis.
Asbestos-related pleural plaques may grow in the absence of continued exposure. However, by themselves, they have no malignant potential. The plaques simply serve as a risk indicator for malignant pleural disease.
Magnetic Resonance Imaging
On magnetic resonance imaging (MRI), most pleural plaques have low signal on T1- and T2-weighted sequences, whereas mesotheliomas are typically T2 hyperintense. Plaques are avascular and do not enhance with contrast.
Positron Emission Tomography–Computed Tomography
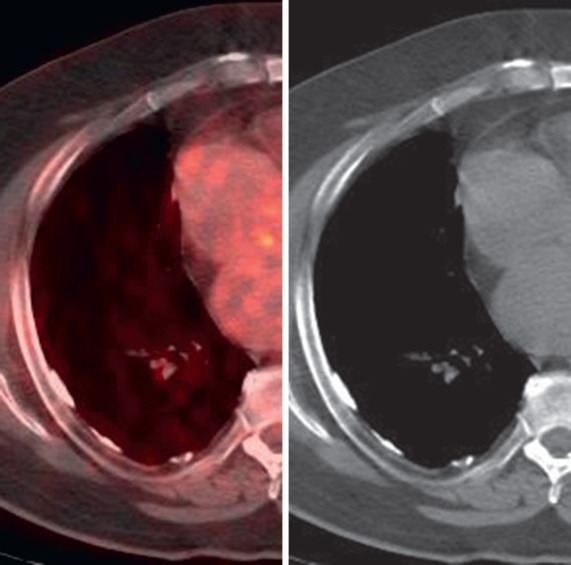
Pleural plaques show no significant uptake of 18 F-fluorodeoxyglucose (FDG) on positron emission tomography (PET) imaging ( Fig. 73.17 ).
Differential Diagnosis
Normal Anatomic Structures
Because the combined thickness of the parietal and visceral pleura is approximately 0.2 mm, the pleura is not visible on the chest radiograph in the normal subject. With CT, a 1- to 2-mm-thick line of soft tissue attenuation is normally seen between the lung and chest wall between the inner edges of the ribs in the intercostal spaces. This line represents the combined thickness of the visceral pleura, normal pleural fluid, parietal pleura, endothoracic fascia, and innermost intercostal muscle. The pleura and endothoracic fascia along the inner aspects of the ribs are too thin to be visible on the CT scan ; however, they may be identified as a thin, smooth line when there is increased extrapleural fat. The intercostal veins should not be confused with the pleura ( Fig. 73.18 ).