, Yuri Peterkin2 and A. Orlando Ortiz2
(1)
Section of Musculoskeletal Radiology, Department of Radiology, Winthrop-University Hospital, Mineola, NY, USA
(2)
Department of Radiology, Winthrop-University Hospital, Mineola, NY, USA
Keywords
Disk aspirationDiskitisEpidural abscessInfectious spondylitisIntervertebral diskOsteomyelitisPercutaneous diskectomyPercutaneous needle biopsySeptic arthritisSpine infection: diagnosisTechnique: coaxialImaging guidance – computed tomographyFluoroscopyLearning
- 1.
To review the clinical evaluation of suspected spine infection
- 2.
To learn the role of image-guided percutaneous spine biopsy during clinical management
- 3.
To introduce specific biopsy techniques and tools for the proper performance of image-guided percutaneous spine biopsy
9.1 Introduction
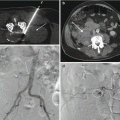
The timely diagnosis and management of patients with spine infection are crucial as delays in diagnosis can cause neurologic impairment and mortality. Infectious spondylitis, or spine infection, is defined as infection of one or more spine structures. The structure or structures of the spine that might become infected include the intervertebral disk, the vertebral body including the vertebral endplate, the posterior elements including the facet joint, the epidural space with possible extension to the subarachnoid space, the spinal cord, and the paraspinal soft tissues (Fig. 9.1).




Fig. 9.1
Spectrum of spine infection. (a) Deep paraspinal muscle infection in an 84-year-old with low back pain and elevated ESR (90) and CRP (200) as shown on fat-suppressed contrast-enhanced T1-weighted axial image. A 56-year-old male with low back pain and fever due to septic left lumbar facet joint (large arrow) with edema in adjacent erector spinae and multifidus muscles (small arrow) as shown on T2-weighted axial image (b), fat-suppressed contrast-enhanced T1-weighted axial image (c), and axial CT image in bone window algorithm (d). Note the juxta-articular erosion within the infected joint (arrow in d). In this 76-year-old female with low back pain and fever, the indium-111 white blood cell study is normal (e), but the T1 sagittal image (f) shows intermediate signal soft tissue (arrow) posterior to the L5 vertebral body and low signal (curved arrow) within the sacral promontory, within hyperintense signal seen within these areas on the T2 sagittal image (g). The fat-suppressed contrast-enhanced T1-weighted sagittal image (h) shows a peripherally enhancing abscess (large arrow) and focal endplate enhancement (small arrows) as well as subtle leptomeningeal enhancement (curved arrows); the epidural abscess (large arrow) is again seen on the fat-suppressed contrast-enhanced T1-weighted axial image (i) as is the leptomeningeal enhancement (curved arrow) consistent with meningitis; deep soft tissue enhancement (small arrow) is also noted
Infectious spondylitis | Infection of one or more of the spine compartments |
Diskitis | Infection confined to the intervertebral disk |
Osteomyelitis | Infection confined to the bone (vertebral body) |
Spondylodiskitis | Infection of the disk and adjacent vertebral bodies |
Septic arthritis | Infection within a facet joint |
Epidural abscess | Epidural space infection with focal purulent collection |
Meningitis | Infection involving the meninges |
Although a relatively less common clinical entity, spine infections are increasing in incidence. Recent studies have reported an estimated increase in the incidence of spine infection from 5.3/100,000 population per year in 2007 to 7.4/100,000 population per year in 2010 (Akiyama et al. 2013). While an improved accuracy in diagnostic capabilities is hypothesized as an etiology for the increased incidence of spine infection, iatrogenic causes also play a significant role. Up to one-third of new cases of vertebral osteomyelitis are healthcare related, and one-third of those cases are secondary to catheter-related infections (Pigrau et al. 2015). Spine surgery is a major risk factor for spine infection (Fig. 9.2). Despite pre-procedure antibiotic prophylaxis, improved surgical techniques, and postoperative care, post-procedural diskitis represents up to 30% of all cases of pyogenic spondylodiskitis (Jiminez-Mejias et al. 1999). Other factors that might account for the increased incidence of spine infection include the increasing age of the overall population, advancements in medicine leading to an increased life expectancy of patients with chronic diseases, and the increased prevalence of patients on immunosuppressive medications (Bhavan et al. 2010; Duarte and Vaccaro 2013; Kim et al. 2015; Pigrau et al. 2015).




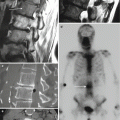
Fig. 9.2
A 40-year-old male with difficulty swallowing after attempted cervical disk procedure. Lateral radiograph (a) of the neck shows extensive prevertebral soft tissue swelling (arrow) with slightly hypointense signal intensity (arrow) on the T1-weighted sagittal image (b) and hyperintensity (arrow) on the T2-weighted sagittal image (c). The fat-suppressed axial image (d) shows mass effect (large arrow) upon the hypopharynx at C4-C5 with a disk herniation that impinges upon the spinal cord (small arrow). The fat-suppressed contrast-enhanced T1-weighted sagittal image (e) shows a heterogeneously enhancing (arrows) retropharyngeal fluid collection which was emergently drained and shown to be an infected hematoma
9.2 Efficacy of Image-Guided Spine Biopsy for Infection
Image-guided percutaneous spine biopsy is a safe and effective procedure with a reported overall accuracy ranging from 88 to 95% (Gupta et al. 2002; Heyer et al. 2008; Rimondi et al. 2008; Tehranzadeh et al. 2007). Although, theoretically, an adequate sample is often obtained with biopsies in patients with suspected spine infection, there is an associated lower overall success rate in identifying the causative organism (Table 9.1). The accuracy for image-guided percutaneous disk space biopsy, in patients with surgically proven spondylodiskitis, has been reported to be as low as 36–57% (Kim et al. 2012; Kim et al. 2015; Marschall et al. 2011; Michel et al. 2006). A negative spine biopsy for spine infection is operationally defined as no evidence of microbial agent growth or identification in the submitted specimen(s) and no histopathologic evidence of diskitis or osteomyelitis in the submitted specimen(s).
Operational definition of a negative biopsy for spine infection:
- 1.
No evidence of microbial identification or growth in the submitted specimen(s)
- 2.
No evidence of disk or vertebral endplate inflammatory change in the submitted specimens
The length of pre-biopsy antibiotic therapy is inversely related to the likelihood of identifying a causative organism and is the most common reason for a false negative biopsy result (Enoch et al. 2008; Kim et al. 2012; Kim et al. 2015; Marschall et al. 2011; Mazzie et al. 2014; Wu et al. 2007). Ideally, a biopsy should be performed before the initiation of antibiotic therapy in order to maximize the probability of obtaining a positive culture result. Alternatively, if the clinical circumstances dictate, then the biopsy should be performed within 48 h of antibiotic administration. A patient who has been placed on antibiotic therapy for a period of time longer than this should have their antibiotic regimen stopped for a minimum of 2 days prior to attempting a biopsy procedure. Other common causes of a false negative biopsy in patients with suspected spine infection include insufficient specimen, improper specimen handling and processing, and obtaining disk material without adjacent subchondral bone (Michel et al. 2006). A repeat spine biopsy in patients with a negative first biopsy and negative blood cultures may yield a positive culture result, and this option, in the appropriate clinical setting, might be considered in patients who are not on antibiotic therapy (Terreaux et al. 2016).
Table 9.1
Reasons for a negative biopsy result in patients with suspected spine infection
1. Patient |
Patient is on concurrent antibiotic therapy |
Incomplete patient work-up in imaging study that mimics spine infection |
Incomplete imaging work-up and analysis prior to performing the biopsy procedure |
2. Procedure |
Unable to access the site of infection |
Wrong level or wrong side is biopsied |
Presence of transitional vertebra |
Abnormality on MRI not well visualized with imaging guidance |
Use of instruments that fail to collect an adequate amount of tissue and fluid |
3. Specimen |
Improper specimen handling |
Insufficient specimen |
Specimen not sent for both microbiologic and pathologic analysis |
The length of pre-biopsy antibiotic therapy is inversely related to the likelihood of identifying a causative organism and is the most common reason for a false negative biopsy result.
9.3 Spine Infection: Mechanisms of Spread
Understanding the pathophysiologic basis of spine infection is integral to perform image-guided percutaneous spine biopsy in patients with suspected spine infection. There are three possible routes of spread that may result in spine infection: (1) hematogenous spread, (2) direct inoculation, and (3) contiguous spread from adjacent structures. Hematogenous spread from a distant site, frequently the genitourinary tract or skin, is the most common cause of spine infection (Bhavan et al. 2010; Diehn 2012; Govender 2005). In adults, hematogenous seeding of vertebral body infection occurs at the level of the end-arterioles adjacent to the subchondral endplates. End-vessel occlusion results in ischemic and necrotic bone; the formation of a bony sequestrum in turn serves as a nidus for progression of infection. Pyogenic infection subsequently spreads from the infected vertebral endplate into the adjacent intervertebral disk (Bhavan et al. 2010; Duarte and Vaccaro 2013; Govender 2005; Jimenez-Mejias et al. 1999). In children, the end-arterioles extend into the intervertebral disk; hence, spine infections in children originate within the disk proper. In adults, therefore, in the setting of suspected vertebral osteomyelitis, disk aspiration and core needle biopsy of the subjacent subchondral vertebral body endplate should both be attempted (Mazzie et al. 2014; Michel et al. 2006). Direct inoculation is frequently due to an iatrogenic etiology. It occurs secondary to spine instrumentation, including spine surgery, lumbar puncture, and percutaneous epidural or facet joint injections. A penetrating injury into or near the spine may also result in direct inoculation. Contiguous spread from an adjacent focus of infection is the least common of the three mechanisms responsible for spine infection. Skin infection (including decubitus ulcers), pulmonary infection, and kidney infection are examples of conditions that can be associated with direct contiguous spread to the adjacent segment of the spine.
9.4 Clinical Presentation
The clinical presentation depends upon two major factors, the virulence of the infectious agent and host resistance factors (Table 9.2). Potential infectious agents include bacterial, mycobacterial, fungal, or parasitic organisms depending on the clinical scenario. Clinically, spine infections are generally challenging to diagnose as patients may present with subtle and non-specific symptoms, which range in acuity. Therefore, a significant delay in clinical diagnosis may occur. A strong clinical suspicion of spine infection should be supported by correlation with pertinent imaging studies and laboratory analysis. On initial presentation, the most common reported symptom is unremitting back pain, which worsens at night and does not dissipate with rest. The lumbar spine is the spinal segment that is most frequently involved. Fever is an unreliable sign of spine infection as up to 54% of patients are afebrile at initial presentation (Bhavan et al. 2010). Neurologic deficits including lower extremity weakness, radiculopathy, and urinary incontinence have been reported in up to one-third of patients and are often associated with delays in diagnosis (Duarte and Vaccaro 2013). Spine infections are more common in males, and the incidence increases with age, most commonly affecting adults who are 50 years of age or older. Predisposing risk factors include intravenous drug abuse, chronic disease such as renal failure or diabetes, previous spinal surgery, or HIV infection or other immunocompromised state (Bhavan et al. 2010; Diehn 2012; Duarte and Vaccaro 2013; Govender 2005).
Table 9.2
Risk factors for spine infection
1. Age greater than 50 years |
2. Intravenous drug use |
3. Pre-existing source of infection |
4. Diabetes |
5. HIV infection or other immunocompromised state |
6. Previous spine surgery |
7. Chronic steroid use |
8. Chronic medical condition (renal failure, cirrhosis) |
A key initial step in diagnosing spine infection is to suspect it!
9.5 Laboratory Findings
There are several serum laboratory markers, which may be helpful in diagnosing and managing spine infection. The erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) are inflammatory markers that are commonly elevated at initial presentation. ESR is a sensitive but non-specific measure of inflammation. It is the rate at which red blood cells layer, or sediment, in 1 h (Singh 2014). ESR directly correlates with the amount of fibrinogen in the blood, increasing with any condition that elevates fibrinogen. Other causes of an increased ESR include pregnancy, anemia, autoimmune disorders, multiple myeloma, and lymphoma. CRP is an acute phase protein of hepatic origin, which rises in response to the release of interleukin-6 by macrophages and T-cells (Go et al. 2012; Singh 2014). Infections and inflammatory diseases are common causes of an increase in serum CRP levels (Heyer et al. 2012). Pregnancy, obstructive sleep apnea, and malignancy can also cause an elevated CRP. Typically in spine infection, both ESR and CRP are elevated at initial presentation. However, bone pathology, specifically in diabetics, is reported as a common factor in causing an elevated ESR with a normal CRP level (Singh 2014). ESR is the most useful marker of inflammation, with elevation reported in 70–100% of infections at presentation (Go et al. 2012). Inflammatory markers are often followed to assess the patient’s response to treatment. Serum CRP returns to normal with treatment faster than ESR and is therefore a better marker for therapeutic response in patients with infection (Brigden 1999; Duarte and Vaccaro 2013; Singh 2014). The white blood cell count (WBC) is the least useful of the inflammatory markers due to its low sensitivity. In a large 2-year retrospective cohort study, 40% of patients who presented with or developed hematogenous vertebral osteomyelitis had a normal initial WBC (Bhavan et al. 2010). Positive blood cultures may be seen in approximately 24% of patients with suspected spine infection and may assist in identifying the offending microorganism and guiding subsequent treatment. In specific situations, when a coagulase-positive Staphylococcus infection is suspected, the use of counterimmunoelectrophoresis to detect serum anti-teichoic acid antibodies may be helpful in confirming the presence of staphylococcal infectious spondylitis (Dhale et al. 2003). Ribitol teichoic acid, found within the cell wall of Staphylococcus aureus species, is antigenic and a high serum titer (> 4) of anti-teichoic acid antibodies which may be detected in patients with staphylococcal spine infection.
9.6 Imaging
Due to the insidious and non-specific clinical presentation of infectious spondylitis, radiologists have an integral role in facilitating this diagnosis. Radiographs are often the initial imaging examination performed; however, plain films have an extremely low sensitivity for detection of early infection and may remain normal for several weeks (Diehn 2012; Govender 2005). Despite the low sensitivity for acute spine infection, radiographs often demonstrate findings of spine infection due to the delayed presentation that is associated with this condition. Loss of cortical definition with irregularity of the vertebral endplate is the earliest radiographic finding in spondylodiskitis (Diehn 2012; Go et al. 2012; Govender 2005). Radiographic detection of bone loss requires a 30–40% loss of the bony matrix typically occurring 2 weeks after initial symptoms (Go et al. 2012). Prevertebral or paraspinal soft tissue swelling, fullness, or bulging with loss of fat planes can also be identified on radiographs in early cases of spine infection (Diehn 2012; Go et al. 2012; Govender 2005). As the infection progresses, there is subsequent involvement of the intervertebral disk space, with loss of disk height and erosive changes of the vertebral endplates (Fig. 9.3). Radiographic findings of chronic infection include sclerosis of the vertebral endplates with variable collapse of the infected vertebral body, obliteration, and fusion across the affected disk space, leading to spinal deformities such as kyphosis and/or scoliosis (Diehn 2012; Go et al. 2012). In chronic spine infection, especially tuberculous spondylitis, calcification may be observed within the paraspinal soft tissues or within the epidural space.




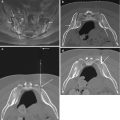
Fig. 9.3
An 11-year-old male with S. aureus proven septic spondylodiskitis. Lateral radiograph (a) of the lumbar spine shows L5-S1 disk space narrowing (arrow) with vertebral endplate erosions and subchondral sclerosis. Reformatted sagittal CT image (b) in bone window algorithm shows irregularity, sclerosis, and erosion of the subchondral bone (arrow) along the L5-S1 endplates. Sagittal T1-weighted image (c) shows obliteration of the disk space with loss of the cortical margins (arrow), while the sagittal T2-weighted image (d) shows hyperintense T2 signal within the disk space, as well as extensive vertebral bone marrow edema (arrow). Sagittal (e) and axial (f) T1-weighted fat-suppressed contrast-enhanced images show intradiskal enhancement with intradiskal abscess (arrows). Axial CT image acquired during biopsy at the level of the L5-S1 disk space (g) shows coaxial advancement of the biopsy needle through the guiding cannula (arrow) via a right S1 transpedicular approach utilizing cranial angulation through the pedicle (P) for successful sampling of the vertebral endplate and the adjacent disk
Computed tomography (CT) has a higher sensitivity than plain radiography for the detection of early bony changes in spine infection due to the increased anatomic resolution. CT findings of spine infection are similar to those seen on radiographs; however, subtle endplate irregularity and erosions are better depicted (Fig. 9.4). Loss of the normal architecture of the trabecular bone is one of the early CT findings of pyogenic vertebral osteomyelitis, which is rarely appreciated on radiographs (Go et al. 2012). CT is commonly utilized in patients with contraindications to magnetic resonance imaging (MRI) and for differentiating mimickers of spondylodiskitis, such as reactive vertebral endplate changes. CT is useful for the depiction of the spread of infection and helps to characterize prevertebral and paraspinal soft tissue involvement. Mass effect from infected paraspinal collections can compromise the neural foramen and may cause nerve root impingement. Posterior extension of infection can involve the epidural space and, in the cervical or thoracic spine, may result in spinal cord compression. In patients that cannot undergo an MRI examination, this study may need to be performed with an intravenous contrast agent or, less commonly, with an intrathecal contrast agent.




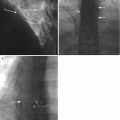
Fig. 9.4
A 53-year-old male with pathological analysis showing acute inflammation and purulent exudates and culture-positive gram-positive cocci in pairs. Reformatted sagittal CT image (a) in bone window algorithm shows irregularity of the inferior endplate of L4 with loss of cortical bone (arrow) and increased intervertebral disk height anteriorly. Sagittal T1-weighted image (b) also shows loss of the normal hypointense line along the inferior endplate of L4 (arrow) as well as hypointense T1 signal adjacent to the vertebral endplates of L4 and L5. Sagittal T2-weighted image (c) shows corresponding bone marrow edema (arrows) and hyperintense signal within the disk (arrowhead). Sagittal T1-weighted fat-suppressed contrast-enhanced image (d) shows prominent endplate enhancement (arrows) and focal epidural enhancement (arrowhead). Axial CT image (e) acquired during biopsy shows the biopsy needle (small arrow) advanced coaxially through a guide cannula (large arrow) via a posterolateral paravertebral approach directly into the L4-L5 disk space
Magnetic resonance imaging is the study of choice for diagnosing spine infection, with a reported sensitivity of 96%, specificity of 92%, and accuracy of 94%. Endplate irregularity, with loss of cortical definition, and erosions are common and may later progress to vertebral body destruction. The earliest MRI finding in spine infection is altered bone marrow signal manifested as hypointense T1- and hyperintense T2-weighted signal with contrast enhancement, most prominent along the vertebral endplates (Fig. 9.4). Involvement of the adjacent intervertebral disk space may manifest with loss of intervertebral disk height, alteration of normal disk morphology including loss of the intranuclear cleft, focal T2 hyperintensity, and variable contrast enhancement patterns (Fig. 9.5). Infection may also spread posteriorly into the epidural space and laterally into the paravertebral soft tissues. Because of the initial involvement of the vertebral endplate, loss of the normal disk-endplate margin may be a helpful sign in suspecting possible infection. Psoas musculature T2 hyperintensity shows a high sensitivity and specificity (92% at a 95% confidence interval) with a high positive likelihood ratio for spondylodiskitis; this may be a helpful imaging finding especially when an unenhanced MRI study is performed and may raise a concern for possible spine infection (Ledbetter et al. 2016). A contrast-enhanced MRI examination is the study of choice to evaluate a patient with a suspected spine infection and/or epidural abscess with possible spinal cord compression (Fig. 9.6). Initially, irregular, thick paraspinal, or epidural soft tissue enhancement is seen compatible with phlegmon. Paraspinal abscesses are readily identified on MRI as T1-hypointense and T2-hyperintense fluid collections with peripheral enhancement. Spine infections however can have a variable appearance on MRI, with atypical imaging characteristics and variable vertebral involvement with sparing of the intervening disk spaces. MRI findings with the reported highest sensitivity for the diagnosis of spine infection are vertebral body T1-hypointense signal, intervertebral disk space T2-hyperintense signal, and disk space enhancement (Diehn 2012) (Fig. 9.7). Epidural abscess formation may be associated with spondylodiskitis or, depending on the etiology (e.g., a spinal procedure), may be seen in isolation (Fig. 9.8). MRI will show a heterogeneous T1-hypointense and T2-hyperintense variable-length fluid collection within the epidural space that is associated with prominent peripheral and epidural contrast enhancement. It should be noted that, at the cervical and/or thoracic spine level, a patient’s myelopathic presentation may be disproportionately greater than the severity of spinal cord compression because the associated spinal cord ischemia also reflects the presence of epidural venous plexus vascular congestion. Untreated epidural abscesses can progress rapidly and cause significant morbidity and mortality. The detection of a suspected epidural abscess should prompt immediate spine surgical consultation for consideration of emergent drainage and decompression of the epidural abscess.





Fig. 9.5
MRI signs of early disk space infection. T1-weighted sagittal image (a) shows loss of the hypointense lines (arrows) that correspond to the vertebral endplate; compare to the normal vertebral endplate at the level above (curved arrow). T2-weighted sagittal image again shows vertebral endplate irregularity/erosion (arrow) and loss of the normal intranuclear cleft (curved arrow). Contrast-enhanced T1-weighted sagittal image (c) shows prominent marrow enhancement and ring enhancement surrounding an intradiskal abscess (arrow)



Fig. 9.6




Chronology of a case of spine infection. Lateral radiograph of the lumbar spine (a) in a patient with acute low back pain is normal. T1-weighted sagittal image (b) obtained on the same day shows hypointense endplate signal (arrow) which was attributed to degenerative endplate change at L5-S1; note the subtle cortical erosion of the endplate (curved arrow). The T2 sagittal image (c) shows reactive endplate edema (large arrow), loss of the intranuclear cleft (small arrow), and thick hyperintense signal (curved arrow) adjacent to the posterior annulus. Three weeks later, a repeat MR examination shows further loss of the normal T1 hypointense endplate signal (d) as compared to the level above (curved arrow) and prominent marrow edema. The T2-weighted sagittal image (e) shows progression of intradiskal signal increase (arrow) with contrast enhancement confined to the endplates and adjacent marrow as shown on the fat-suppressed contrast-enhanced T1-weighted image (f). The findings were attributed to degenerative disk disease with reactive endplate change at L5-S1, and conservative medical management was continued. The patient’s back pain symptoms persisted, and lateral radiograph (g) obtained 10 weeks after the initial onset of the patient’s symptoms shows complete loss of the cortical endplates at L5-S1 (arrows); compare to the normal level above (curved arrow). A third MRI study obtained 12 weeks from the onset of symptoms now shows extensive marrow edema and disk space height loss with disorganization and signal abnormality with extensive vertebral body and intradiskal enhancement at L5-S1 as shown on the sagittal T1(h), T2 (i), and fat-suppressed contrast-enhanced T1-weighted (j) images
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree