Bladder Carcinoma
Todd M. Blodgett, MD
Alex Ryan, MD
Omar Almusa, MD
Key Facts
Imaging Findings
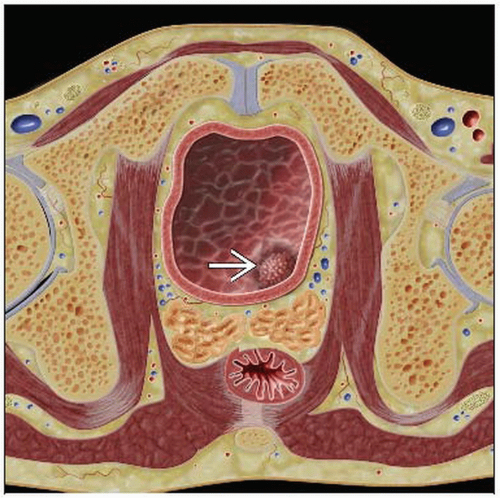
CT: Enhancing focal/asymmetrical mass in the urinary bladder
Can be multifocal
Lymphatic spread (30% of tumors that only involve bladder wall; 60% of those with extravesicular invasion)
Common metastatic sites include pelvic and retroperitoneal lymph nodes, lungs, liver, and bones
Initial workup: Cystoscopy and biopsy; CT or MR for evaluation of primary tumor, LN metastases
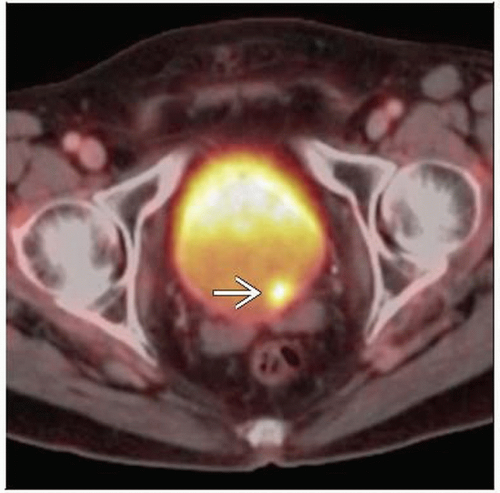
PET/CT: Valuable for pre-operative staging, response to therapy, distinguishing post-surgical change from recurrence
CT findings nonspecific, diagnosis usually based on biopsy
Focal or diffuse bladder wall thickening
Mass projecting into bladder ± enhancement
Pathology
˜ 90% transitional cell carcinomas (TCC); often multifocal
Clinical Issues
Hydronephrosis, renal obstruction, particularly with lesions near UVJ
Diagnostic Checklist
Bladder cancer may have variable FDG uptake; baseline PET/CT useful to confirm FDG avidity
Immediate post-void imaging, retrograde bladder irrigation with normal saline, IV Lasix administration with parenteral hydration have been recommended
TERMINOLOGY
Abbreviations and Synonyms
Bladder carcinoma, urothelial carcinoma, transitional cell carcinoma (TCC)
Definitions
Malignancy of the urinary bladder
IMAGING FINDINGS
General Features
Best diagnostic clue
PET/CT: Focal increased FDG activity in primary tumor, ± in regional and distant lymph nodes, lung, liver, bone
CT: Enhancing focal/asymmetric mass in the urinary bladder
Location
Usually arises in the bladder wall
Can be multifocal
Local invasion
Detrusor muscle, prostate, uterus, vagina, seminal vesicles, rectum
Lymphatic spread (30% of tumors that only involve bladder wall; 60% of those with extravesicular invasion)
Regional (pelvic) lymph nodes (LN)
Distant LN
Hematogenous spread
Lung > > liver, bone
Recurrences of superficial bladder cancer remain confined to bladder wall in 70-80% of patients
Remaining 20-30% may become muscle-invasive and lead to metastatic disease
Common metastatic sites include pelvic and retroperitoneal lymph nodes, lungs, liver, and bones
Size: Varies from undetectable on CT to large enhancing mass
Morphology
Imaging Recommendations
Best imaging tool
Initial workup: Cystoscopy and biopsy; CT or MR for evaluation of primary tumor and LN metastases
CT: Reported accuracy in detecting LN involvement 70-90% with false negative rates 25-40%
MR: 73-98% reported accuracy for determining nodal metastases
PET/CT
Utility mostly for pre-operative staging, distinguishing post-surgical change from recurrence
Minimally useful for evaluation of primary tumor, usually obscured by excretory FDG
Bone scan: Helpful if there is clinical suspicion of bone metastases
CT/MR tend to overestimate degree of extension through bladder wall but underestimate presence of pelvic lymph node metastases
Protocol advice
PET/CT: 10-15 mCi (370-555 MBq) F18-FDG IV, start imaging at pelvis to avoid FDG filling bladder
PET/CT: Excreted FDG in urinary bladder can mask pelvic pathology
Techniques include Immediate post-void imaging, retrograde bladder irrigation with normal saline, IV Lasix administration with parenteral hydration
Prone positioning may be useful for visualization of posteriorly located lesions
Urinary excretion of FDG leads to pooled activity in bladder, making evaluation of bladder wall lesions difficult to impossible with standard protocol
Furosemide injected at least 2 hours after radiotracer injection provides excellent urinary radiotracer washout, reducing bladder activity to background levels
Oral hydration aids in diuresis
Full bladder is required to avoid artifactual thickening of the walls
Protocol not satisfactory in patients with cystectomy because urinary diversions show higher residual activities, but recurrence is extremely rare in bladder diversion walls
CT Findings
CT findings of primary usually nonspecific (diagnosis often based on biopsy performed during cystoscopy)
Focal or diffuse bladder wall thickening
Mass projecting into bladder ± enhancement
Occasionally calcifications
Hydronephrosis 2° to tumor near vesicoureteric junction
Extravesicular extension: Nodules, irregularity of outer bladder wall, stranding of perivesicular fat
T status of tumor most often determined by biopsy
Causes of circumferential bladder thickening that can mimic bladder cancer include
Previous biopsy, inflammation, radiotherapy, systemic chemotherapy, and intravesical agents like Bacille Calmette-Guérin (BCG)
Sessile or pedunculated soft tissue mass projecting into the lumen; similar density to bladder wall
± Enlarged (> 10 mm) metastatic lymph nodes; extravesical tumor extension
Fine punctate calcifications with tumor; may suggest mucinous adenocarcinoma
Ring pattern of calcification; may suggest pheochromocytoma
Inability to distinguish tumors from bladder wall hypertrophy, local inflammation, and fibrosis
Unable to differentiate Ta-T3a, invasion of dome/base of bladder or local organ (due to partial volume effect), nonenlarged lymph nodes
Also consider urachal adenocarcinoma
Midline abdominal mass ± calcification
Solitary lobulated tumor arising from dome of bladder on ventral surface
MR Findings
T1WI: Isointense to muscle
T2WI: Hyperintense to muscle
Superior to CT for assessing deep muscle involvement, invasion of adjacent organs
Nuclear Medicine Findings
Focal increased FDG activity in primary tumor, regional and distant LN, lung, liver, bone
Bladder cancer may have variable uptake of FDG
Baseline PET/CT useful for confirmation of FDG avidity
Max SUV may range from 5-10 in typical hypermetabolic bladder lesions
Primary tumor may be masked by excreted FDG in urine
FDG is not resorbed as glucose and is excreted in the urine
With delayed imaging, ratio of tumor:bladder FDG uptake was 13:1 in one study
False positives: Bladder diverticula and urinary leak
CT portion of PET/CT essential for ruling out such pitfalls
CT also useful for precise separation of uptake foci in the bladder wall vs. lymph nodes adjacent to bladder
One study showed 3 month period post-resection was sufficient to heal inflammatory reactions and reduce false positives
C-11-choline PET/CT radiotracer does not collect in urinary tract, but its value for lesion staging has not been shown superior to conventional methods
Bone scan: Bone metastases classically appear as multiple, scattered foci of increased activity, axial > appendicular skeleton
DIFFERENTIAL DIAGNOSIS
Cystitis
Usually more diffuse thickening of the bladder wall
Chronic urinary tract infection, fungus
Radiation- or chemotherapy-induced
Hemorrhagic cystitis
Hematoma
Trauma
Iatrogenic
Cystitis Cystica
Degeneration of urothelial cells in Brunn nests
Other Neoplasm
Endometriosis
Metastases
PATHOLOGY
General Features
General path comments
˜ 90% transitional cell carcinomas (TCC); often multifocal
5-10% squamous cell (chronic inflammation)
< 5% mixed TCC and squamous cell
2-3% adenocarcinoma (persistent urachal remnant)
< 1% rare types (e.g., leiomyomas, lymphoma, melanoma)
Etiology
Risk factors
Cigarette smoking
Exposure to aniline, aromatic amines, diesel fumes
Phenacetin use (once used as analgesic, now often mixed with cocaine)
Infection: Chronic urinary tract infection, schistosomiasis
Epidemiology
Most common tumor of urinary tract
More than 90% are transitional cell carcinoma
Men: Fourth most common cancer
7% of all malignancies in men
Women: Tenth most common cancer
2% of all malignancies in women
Staging, Grading, or Classification Criteria
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree