2 Bone and Soft-tissue Infections Septic arthritis and spondylitis/diskitis are discussed in Chapter 9 (pages 331–337). Organisms: Route of infection: The necrotic tissue can be resorbed and replaced with new bone. If this fails, purulent cavities (abscesses) or sequestra form. Acute osteomyelitis is treated with high doses of antibiotics. The major role of imaging is to identify abscesses or sequestra since they are not adequately treated by antibiotics and often require decompression or surgical intervention. Newborn osteomyelitis is generally an acute disease and has a predilection of the proximal and distal femoral metaphyses. Predisposing conditions include infections of the umbilicus, ear, nose, and throat, and the most common organisms are streptococci. If untreated, the infection spreads rapidly through the Haversian canals into the subperiosteal space or across the still patent meta-epiphyseal vessels to the epiphysis (Figs. 2.2, 2.3). The result is a septic arthritis with capsular empyema, destruction of the epiphyseal cartilage, and epiphyseal separation, ultimately causing severe joint deformity if not aggressively treated.
Osteomyelitis
 Osteomyelitis is diagnosed by integrating clinical, laboratory, and imaging findings. Isolating the responsible organism is desirable for testing antibiotic sensitivity, but succeeds in only a portion of cases. Establishing the diagnosis ‘osteomyelitis’ by open or percutaneous biopsy is rarely necessary today.
Osteomyelitis is diagnosed by integrating clinical, laboratory, and imaging findings. Isolating the responsible organism is desirable for testing antibiotic sensitivity, but succeeds in only a portion of cases. Establishing the diagnosis ‘osteomyelitis’ by open or percutaneous biopsy is rarely necessary today.
 The infection arises as the organisms spread in to the perivascular interstitial structures, leading to a leukocytic infiltration that permeates the bone marrow. The dissemination progresses along the Volkmann’s canals and through the Haversian canals. The infiltration leads to vascular compression, compromises the nutrition of the bone marrow and, together with the bacterial toxins, ultimately causes ‘necrosis’.
The infection arises as the organisms spread in to the perivascular interstitial structures, leading to a leukocytic infiltration that permeates the bone marrow. The dissemination progresses along the Volkmann’s canals and through the Haversian canals. The infiltration leads to vascular compression, compromises the nutrition of the bone marrow and, together with the bacterial toxins, ultimately causes ‘necrosis’.
Acute Hematogenous Osteomyelitis
Newborn Osteomyelitis
| Nonspecific osteomyelitis | Specific osteomyelitis | |
|---|---|---|
| Acute types | Chronic types | |
| Hematogenous osteomyelitis (newborn, child, adult) | Primary (endogenous) | Mycobacterium tuberculosis |
| – Chronic hematogenous osteomyelitis | Salmonella | |
| – Brodie abscess | Treponema pallidum | |
| – Chronic recurrent multifocal osteomyelitis | Fungi and others | |
| –Plasma cellular osteomyelitis | ||
| Secondary (exogenous) |
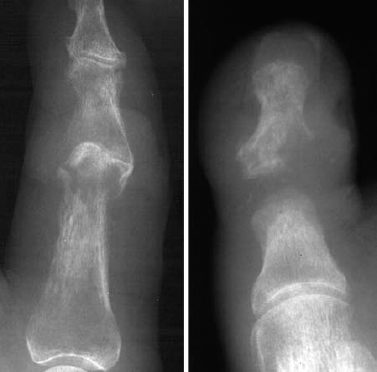
Fig. 2.1 Two different examples of osteomyelitis spread from contiguous soft-tissue infection.
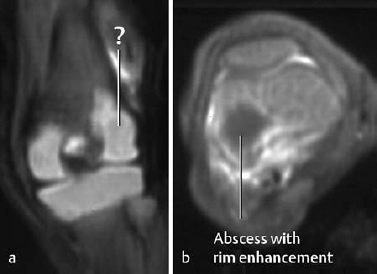
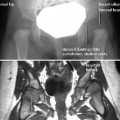
Fig. 2.2 Osteomyelitis in an 18-day-old newborn with abscess in the femoral epiphysis. The coronal T1-weighted sequence (a) fails to delineate the abscess clearly. The abscess, however, is distinctly displayed after enhancement on the axial T1-weighted image with fat suppression (b).
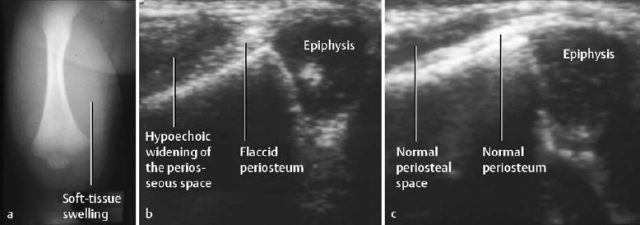
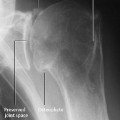
Fig. 2.3 Acute osteomyelitis in an 11-day-old newborn with extension along the subperiosteal space. There is considerable soft-tissue swelling (a). Sonography (b) shows periosteal and parosteal changes, more readily appreciated by comparing the findings with the normal contralateral site (c).
Juvenile Hematogenous Osteomyelitis
Beginning in the second year of life, metaphyseal vessels no longer penetrate the growth plate and form dilated loops in the metaphysis. This vascular change provides favorable conditions for infective organisms and explains the development of metaphyseal inflammatory foci. This absence of vascular penetration of the growth plate retards the spread of infection to the epiphysis (epiphyseal barrier). At this time the suppurative process generally penetrates the thin cortex rapidly and spreads along the subperiosteal space, elevating the periosteum. The infection then spreads into the joint only if the metaphysis is intra-articular, as in the hip and knee.
The organisms causing juvenile osteomyelitis are predominantly staphylococci, especially Staphylococcus aureus.
 In this condition the patient generally presents with sudden onset of fever, chills, severe pain, and extreme local tenderness. The major clinical findings are restriction of motion of the affected extremity usually due to pain, local swelling, erythema, and hyperthermia with elevation of inflammatory parameters.
In this condition the patient generally presents with sudden onset of fever, chills, severe pain, and extreme local tenderness. The major clinical findings are restriction of motion of the affected extremity usually due to pain, local swelling, erythema, and hyperthermia with elevation of inflammatory parameters.
Acute Hematogenous Osteomyelitis in the Adult
Acute hematogenous osteomyelitis in the adult is relatively rare and primarily affects flat bones, vertebral bodies, and the diaphysis of the tubular bones. The suppurative process can involve the entire medullary space (medullary phlegmon).
Vascular connections between the metaphysis and epiphysis are restored after closure of the growth plate, and there is subchondral extension of the vessels. This modification of vascular flow allows spread of the infection into the joint capsule more frequently. Since the metaphyseal cortex is thick and the overlying periosteum fibrosed and firmly attached, subperiosteal abscesses rarely develop. In the rare cases of cortical penetration, the suppurative process breaks through the rather rigid periosteum and spreads into the soft tissues, resulting in the formation of fistulous tracts. Sequestration is rare.
 The local findings (swelling, pain, tenderness) are associated with occasionally severe systemic findings (fever, weakness). The inflammatory parameters (complement fixation tests, sedimentation rate, leukocytes) are generally elevated.
The local findings (swelling, pain, tenderness) are associated with occasionally severe systemic findings (fever, weakness). The inflammatory parameters (complement fixation tests, sedimentation rate, leukocytes) are generally elevated.
Imaging Features of Acute Hematogenous Osteomyelitis (All Ages)
 Numerous radiographic findings have to be considered:
Numerous radiographic findings have to be considered:
- The types and extent of medullary destruction are variable. The entire destructive spectrum can be encountered, including a solitary radiolucency (Fig. 2.4), irregular, multiple radiolucencies (‘mottling’ or permeative pattern), and homogeneous bone loss.
- The lesions are generally indistinct and irregular in outline.
- In contrast to those in newborns and infants, the soft-tissue findings in adults are rarely diagnostically helpful.
- Lamellated periosteal reactions are invariably present (Fig. 2.5).
- The reparative phase during therapy is characterized by endosteal and periosteal new bone formation, development of a surrounding sclerosis, and even large osteosclerotic areas.
- In the newborn and infants, the loss of normal fat planes on conventional radiographs might be an early sign of soft-tissue swelling (within days of the onset of clinical findings). It takes about 7 to 10 days for the osseous findings to become radiographically visible, whereby the lamellated periosteal changes are generally discernible before any bone destruction. A late manifestation in this age group is the ‘ballooned metaphysis’, with possible involvement of the epiphysis. Late in the disease, a strong periosteal reaction can appear as periosteal ossification.
 The radionuclides used for diagnosing osteomyelitis can be divided into bone-seeking tracers (e.g., Tc 99 m diphosphonate) and inflammation-avid tracers (e.g., Tc 99 m-labeled leukocytes).
The radionuclides used for diagnosing osteomyelitis can be divided into bone-seeking tracers (e.g., Tc 99 m diphosphonate) and inflammation-avid tracers (e.g., Tc 99 m-labeled leukocytes).
Three-phase Bone Scan with Tc 99 m diphosphonate
The osseous accumulation of diphosphonate depends on:
- regional blood flow,
- regional distribution of sympathetic innervation,
- osseous turnover at the site of the inflammation.
The three phases of the examination are:
- vascular phase, representing the initial passage of the tracer, of about 1 minute,
- blood pool phase, corresponding to the first 2–4 minutes after the injection of tracer,
- static or bone phase, about 2–4 hours after the injection of tracer.
Acute osteomyelitis produces intense focal radionuclide accumulation in all three phases. Increased blood pool activity without osseous uptake on the static images suggests an inflammation confined to the soft tissues (Fig. 2.6).
Cautionary Note: In the newborn, acute osteomyelitis can lead to a decreased uptake, resulting in a false-negative bone scan or a ‘photon-deficient’ lesion. This has been attributed to increased intramedullary pressure. Another false-negative finding can be caused by the inability to discern increased radionuclide uptake of inflammation because of the accentuated activity of the growth plates.
Tc 99 m-labeled WBC Scan
Two labeling techniques can be used:
- HMPAO to label a suspension of autologous leukocytes with Tc 99 m in vitro.
- Murine monoclonal antibodies for in vivo labeling of granulocytes, with the antibodies not only binding to the granulocytes but also diffusing through the capillary walls into the inflammatory tissue.
Any tracer accumulation outside the expected physiologic pattern suggests an osteomyelitis. Whether alone or combined, the WBC scan will increase the specificity but not affect the sensitivity of detecting inflammation. Since nonspecific WBC accumulation can occur in tumors and fractures, correlation with current conventional radiographs is necessary.
Ga 67 scanning no longer plays a major role in diagnosing infection because of its high radiation dose and low specificity. Nanocolloid scanning has been abandoned because of its low sensitivity.
 The acoustic properties of the neonatal anatomy are especially suitable for sonography The first sonographic sign, seen even before any periosteal reaction, is deep soft-tissue edema and swelling. This is followed by sonographic detection of a thin hypoechoic fluid layer, which elevates the periosteum and may progress to a space-occupying abscess if the infection is not adequately treated. Sonography however, should not be overrated since these ultrasonographic findings are not always detectable. Depending on the site examined, the false negative rate can be as high as 40%. Moreover, sonographic findings are often nonspecific and can be falsely interpreted as evidence of osteomyelitis.
The acoustic properties of the neonatal anatomy are especially suitable for sonography The first sonographic sign, seen even before any periosteal reaction, is deep soft-tissue edema and swelling. This is followed by sonographic detection of a thin hypoechoic fluid layer, which elevates the periosteum and may progress to a space-occupying abscess if the infection is not adequately treated. Sonography however, should not be overrated since these ultrasonographic findings are not always detectable. Depending on the site examined, the false negative rate can be as high as 40%. Moreover, sonographic findings are often nonspecific and can be falsely interpreted as evidence of osteomyelitis.
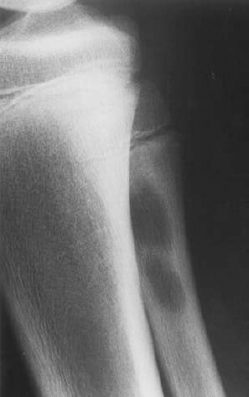
Fig. 2.4 Acute osteomyelitis of the proximal fibula, manifested as multiple radiolucencies.
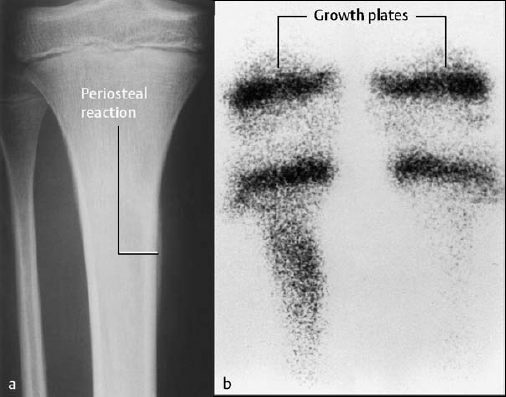
Fig. 2.5 Acute osteomyelitis of the right tibia. The slightly underexposed radiograph (a) reveals periosteal reaction. The static image (osseous phase) of the bone scan (b) shows increased uptake in the proximal right tibia compared to the contralateral side.
Furthermore, sonography can detect a joint effusion that might explain the pain and restriction of motion. This finding frequently prompts a diagnostic joint aspiration in this clinical setting.
 Examination Technique: The most suitable sequences for screening are the STIR (short time inversion recovery) sequences because of excellent visualization of the inflammatory edema, attained by combining T1 and T2 weighting with fat signal suppression. The STIR images are supplemented by T1-weighted SE or fast-spin-echo images, which provide excellent anatomic detail. The echo times of the T2-weighted images should exceed 100 msec for an excellent contrast between inflammation and bone marrow. T2-weighted ‘turbo’ SE sequences should be avoided since they tend to mask any intramedullary edema, unless the contrast can be augmented by frequency-selected fat suppression. Unless contraindicated, intravenous gadolinium-based contrast medium should always be administered since only enhancement can differentiate abscesses or necrotic areas (sequestra) from inflammatory edema. Furthermore, enhancement may exclude a neoplastic process.
Examination Technique: The most suitable sequences for screening are the STIR (short time inversion recovery) sequences because of excellent visualization of the inflammatory edema, attained by combining T1 and T2 weighting with fat signal suppression. The STIR images are supplemented by T1-weighted SE or fast-spin-echo images, which provide excellent anatomic detail. The echo times of the T2-weighted images should exceed 100 msec for an excellent contrast between inflammation and bone marrow. T2-weighted ‘turbo’ SE sequences should be avoided since they tend to mask any intramedullary edema, unless the contrast can be augmented by frequency-selected fat suppression. Unless contraindicated, intravenous gadolinium-based contrast medium should always be administered since only enhancement can differentiate abscesses or necrotic areas (sequestra) from inflammatory edema. Furthermore, enhancement may exclude a neoplastic process.
Morphology and Signal Pattern: The affected areas are low signal intensity on the T1-weighted images with a corresponding area of high signal intensity on the STIR sequence. These areas are often large and irregularly outlined. As a general rule, a periosseous edema is consistently present, and a delicate high-signal rim should be looked for on axial sections. This rule, however, applies to STIR or T2-weighted TSE images with fat suppression only. As long as high-resolution coils are used, STIR and T2-weighted images invariably reveal intracortical areas of increased signal intensity.
Intraosseous abscesses generally are sharply demarcated and display a low-signal rim on the T2-weighted fast-spin-echo and STIR images. A strong peripheral contrast enhancement without any central contrast enhancement supports the presence of an abscess (Fig. 2.7). Necrotic sequestra also fail to show contrast enhancement, but can be differentiated on the T2-weighted or STIR image. In contrast to an abscess, the sequestrum shows a signal void on these images.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


 Osseous infection primarily confined to the bone marrow is a local manifestation of a systemic infection and referred to as hematogenous osteomyelitis. It must be differentiated from secondary osteomyelitis, an osseous infection following trauma or surgery, which might present as osteitis without bone marrow involvement (
Osseous infection primarily confined to the bone marrow is a local manifestation of a systemic infection and referred to as hematogenous osteomyelitis. It must be differentiated from secondary osteomyelitis, an osseous infection following trauma or surgery, which might present as osteitis without bone marrow involvement (
 Acute hematogenous osteomyelitis occurs primarily in the pediatric age group, but its incidence also increases after 50 years of age. Depending on the age of manifestation, a distinction between newborn osteomyelitis, juvenile hematogenous osteomyelitis, and adult osteomyelitis is usually made.
Acute hematogenous osteomyelitis occurs primarily in the pediatric age group, but its incidence also increases after 50 years of age. Depending on the age of manifestation, a distinction between newborn osteomyelitis, juvenile hematogenous osteomyelitis, and adult osteomyelitis is usually made. 
 The typical acute clinical course consists of fever, restriction of motion often due to pain, severe point tenderness, and elevated inflammatory parameters. Soft-tissue swelling, erythema, and increased skin temperature develop relatively late in the clinical course.
The typical acute clinical course consists of fever, restriction of motion often due to pain, severe point tenderness, and elevated inflammatory parameters. Soft-tissue swelling, erythema, and increased skin temperature develop relatively late in the clinical course.