Bone and Soft Tissue
Thomas F. DeLaney
David G. Kirsch
RADIATION THERAPY FOR SARCOMAS
Contemporary management of sarcomas often includes a multidisciplinary approach using a combination of surgery, radiotherapy, and chemotherapy specific for tumor type, histologic grade, and stage of disease. Because these tumors are relatively uncommon and present in a variety of anatomic locations, most clinicians see these tumors infrequently. However, because they can often be successfully treated with good functional outcome by sarcoma centers with appropriate multidisciplinary expertise, referral to centers with experienced sarcoma teams is the most appropriate management strategy.
Radiotherapy can be employed as neoadjuvant (preoperative), adjuvant (postoperative), or primary local therapy depending upon the site and type of tumor, the availability and acceptability of the surgical option, and the efficacy of the chemotherapy. Neoadjuvant (preoperative) radiotherapy is frequently employed for large, deep soft tissue sarcomas1 and can also be delivered before resection of spine2 or pelvic sarcomas.3 Adjuvant radiation is employed in many centers following resection of soft tissue sarcomas if tumor, or surgically contaminated tissues in patients with incomplete excision, cannot be excised with a minimum of 1 cm of healthy tissue or an intact fascial plane.4 Adjuvant radiation is also employed for patients with bone sarcomas with positive or inadequate margins and in selected other situations that might include presentation with a pathologic fracture,5 poor histologic response to chemotherapy,6 or intralesional excision of or intramedullary rod placement through a radiographically or cytologically benign-appearing lesion later found to be sarcoma on review of final pathologic material. Radiotherapy as the primary local therapy without surgery or in conjunction with subtotal resection is used for medically inoperable patients with soft tissue sarcomas,7,8 for patients with axial Ewing’s sarcomas or extremity Ewing’s sarcomas where surgery would compromise function,9 and for patients with primary bone tumors involving the upper sacrum,10 portions of the pelvis, the base of skull, and the ethmoid/sphenoid sinus region.11
The radiation sensitivity of soft tissue sarcomas appears to be similar to that of breast and other epithelial tissues. Preoperative neoadjuvant radiation therapy (RT) doses of 50 Gy or postoperative adjuvant RT doses of 60 Gy are associated with local tumor control rates in excess of 90% in patients with negative surgical margins. RT provides local control in approximately 75% of patients with soft tissue sarcomas resected with positive margins; doses of 66 to 68 Gy are recommended, as doses of >64 Gy are associated with higher rates of local control and reported to be as high as 85% in one series.12 Ewing’s sarcomas are quite radiation sensitive, and the original description of this tumor by James Ewing’s made note of the fact that the radiation sensitivity of this tumor was one of the features distinguishing it from other bone sarcomas.13 Unresected tumors or gross residual disease is usually treated with 55.8 Gy in association with chemotherapy.9 Consideration of higher doses for high risk bulky axial tumors is reasonable. Microscopic residual disease is usually treated to 50.4 Gy. Chondrosarcomas and osteogenic sarcomas require doses of approximately 66 Gy for control of microscopic residual disease and doses of ≥70 Gy for control of gross residual disease. Chordomas require doses of approximately 70 Gy for microscopic residual disease and doses of >75 Gy for control of gross residual disease.14
RT is most commonly given by externally directed beams but can also be given by brachytherapy or intraoperative techniques. Brachytherapy has been most extensively employed in the adjuvant radiation of soft tissue sarcomas.15 More recently it has been applied on the dura and paraspinal tissues for spine and paraspinal tumors2,16,17 and for some Ewing’s sarcomas with inadequate surgical margins.18 Intraoperative radiotherapy with electron beam
or orthovoltage is delivered to the tumor or tumor bed at the time of surgery. It can be particularly useful to boost the dose to retroperitoneal,19 paraspinal and spinal, and pelvic sarcomas.20 Brachytherapy and intraoperative radiotherapy, although technically challenging, have been adopted in many specialized centers because of their dosimetric advantages over conventional external beam therapy.
or orthovoltage is delivered to the tumor or tumor bed at the time of surgery. It can be particularly useful to boost the dose to retroperitoneal,19 paraspinal and spinal, and pelvic sarcomas.20 Brachytherapy and intraoperative radiotherapy, although technically challenging, have been adopted in many specialized centers because of their dosimetric advantages over conventional external beam therapy.
Because RT for sarcomas often requires high doses in close proximity to sensitive normal tissues, protons and other charged particles are an excellent treatment option for these patients when external beam irradiation is part of the radiotherapy treatment plan. Sarcomas of the skull base and cervical spine were among the first tumors to be treated with protons on a concerted basis and one of the anatomic sites at which excellent clinical results have been achieved with this modality. The charged particle experience with skull base and cervical spine tumors is discussed in Chapter 14. The present chapter will review the experience with bone and soft tissue sarcomas in other anatomic locations. Much of the discussion will focus on protons, but interesting results have also been reported with heavier charged particles, initially helium and neon from Berkeley and more recently carbon ions from the National Institute of Radiological Sciences (NIRS) in Chiba, Japan, and the Gesellschaft für Schwerionenforschung (GSI), Darmstadt, Germany.
SARCOMAS OF THE SPINE AND PARASPINAL SOFT TISSUES
Proton Radiotherapy
Because of the proximity of the spinal cord, radiotherapy for treatment of sarcomas of the spine is constrained by the radiation tolerance of the spinal cord, which is well below that necessary to reliably control most sarcomas in the setting of close or positive margins12 or gross residual disease.8 Proton radiotherapy, with its ability to spare the spinal cord and/or cauda equina and adjacent normal tissues such as the kidney, lung, heart, esophagus, and bowel offers advantages for treatment of tumors in this location. The bone tumor histologies involving the spine include chondrosarcomas, chordomas, osteosarcoma, Ewing’s sarcoma, malignant fibrous histiocytomas (MFH) of bone, fibrosarcomas, and giant cell tumors.
Isacsson et al. compared 3-D conformal radiotherapy treatment plans with photons and protons for a patientwith a spinal Ewing’s sarcoma. Even when only the final 20% of the treatment, the boost to the gross disease, was given with protons, they noted a 5% improvement in local control for a comparable predicted risk of spinal cord injury.21
Hug et al. presented results on combined photon/proton treatment of 47 patients with osteo- and chondrogenic tumors of the axial skeleton.10 Radiation was delivered postoperatively in 23 patients, pre- and postoperatively in 17, and as sole treatment in 7 patients. Mean radiation doses of 73.9 Cobalt-Gray equivalent (Gy [RBE]) 69.8 Gy (RBE), and 61.8 Gy (RBE) were delivered to group 1 (20 patients with recurrent/primary chordoma or chondrosarcoma), group 2 (15 patients with osteogenic sarcomas), and group 3 (12 patients with giant cell tumors, osteo- or chondroblastomas) respectively. Five-year actuarial local control and survival for patients with chondrosarcoma was 100% and 100% and with chordoma was 53% and 50%. Actuarial 5-year local control for patients with osteosarcoma was 59%. The 5-year actuarial local control and survival for the group 3 patients were 76% and 87%. Overall, improved local control was noted for primary versus recurrent tumors, gross total resection, and target doses >77 Gy (RBE).
The Massachusetts General Hospital (MGH) group recently reported on the results of treatment of 16 primary and 11 recurrent sacral chordoma patients managed with high-dose proton/photon treatment alone (6 patients) or combined with surgery (21 patients).14 There was a large difference in local failure rate between patients treated for primary and recurrent chordomas. Local control results by surgery and radiation were 12/14 for primary versus 1/7 for recurrent lesions. Local control results in marginnegative patients were 2 of 3 in the primary and 1 of 2 in the recurrent chordoma groups. For the margin-positive patients, local control results were 10 of 11 and 0 of 5 in the primary and recurrent groups, respectively. Mean follow-up on these locally controlled patients was 8.8 years (including four patients followed for 10.3, 12.8, 17, and 21 years). Radiation alone was used in six patients, four of whom received 73.0 Gy (RBE) or more; local control was observed in three of these four patients for 2.9, 4.9, and 7.6 years. These data indicate a high local control rate for surgical and radiation treatment of primary (12 of 14) as distinct from recurrent (1 of 7) sacral chordomas. Three of four chordomas treated by 73.0 Gy (RBE) or more of radiation alone had local control; one was at 91 months. This indicates that high-dose proton therapy offers an effective treatment option for these patients.
DeLaney et al.,22 recently reported on 50 patients entered between December 1997 and March 2005 onto a prospective, phase II study of High Dose Proton/Photon Radiation Treatment ± Surgical Resection of Sarcomas of the Thoracic/Lumbar Spine/Sacrum and Paraspinal Soft Tissues. Forty-seven of the 50 patients had primary spine sarcomas and 3 patients had paraspinal tumors. Treatment consisted of maximal resection and photon (≤50.4 Gy photon component)/proton radiotherapy; selected patients with high-grade tumors received chemotherapy. Adriamycin was not given concurrent with radiotherapy. Shrinking field technique was used to deliver 50.4 Gy (RBE) to subclinical microscopic disease, 70.2 Gy (RBE) to residual microscopic disease in the tumor bed, and 77.4 Gy (RBE) to gross disease at 1.8 Gy (RBE) q.d.. Doses were reduced by 8% to 10% if chemotherapy was given or diabetes or connective tissue disorders were present. For giant cell tumors or Ewing’s sarcomas, doses did not exceed 61.2 Gy (RBE). Spinal cord
dose was limited to 63/54 Gy (RBE) at surface/center; no specific dose constraint was placed on the cauda equina; but dose was limited to nerve roots and the cauda, especially contralateral to the tumor, if possible. If surgery was performed at our institution, preoperative RT of 19.8 Gy (sacrococcygeal) or 50.4 Gy (thoracolumbar) was given; when possible, intraoperative, dural brachytherapy boost was given with a customized Yttrium-90 (90Y) plaque.2
dose was limited to 63/54 Gy (RBE) at surface/center; no specific dose constraint was placed on the cauda equina; but dose was limited to nerve roots and the cauda, especially contralateral to the tumor, if possible. If surgery was performed at our institution, preoperative RT of 19.8 Gy (sacrococcygeal) or 50.4 Gy (thoracolumbar) was given; when possible, intraoperative, dural brachytherapy boost was given with a customized Yttrium-90 (90Y) plaque.2
Histologies included chordoma (29 patients), chondrosarcoma (14 patients), and, in one patient each, liposarcoma, osteosarcoma, Ewing’s sarcoma, giant cell tumor, angiosarcoma, malignant schwannoma, and spindle cell sarcoma. Twenty-six patients had sacral lesions, 13 had lumbar lesions, and 11 had thoracic lesions. Thirty-six patients were treated at the time of primary presentation and 14 were treated for tumor locally recurrent after prior surgery. As part of their treatment on protocol, 25 patients underwent gross total resection of their tumor, with positive margins in 17 patients and negative margins in 8 patients. Tumor was subtotally excised in 12 patients and just biopsied in 13 patients. For patients undergoing biopsy only, the median size of the tumor was 7 cm (median 3 to 20 cm). One patient chose to not complete RT for social reasons. Otherwise, RT was given per protocol within 3% of specified dose, with the shortfall driven by the spinal cord dose constraint. The median radiation dose was 76.6 Gy (RBE) (range 59.4 to 77.4 Gy [RBE]). Three patients received a 10 Gy boost to the dural surface with a 90Y dural plaque.
With median follow-up after start of RT of 34 months, six patients have suffered local tumor recurrence (4 chondrosarcomas and 2 chordomas, ρ = 0.016) to yield a 3-year actuarial local control rate of 87%. Two of the chondrosarcoma patients who recurred after proton RT had suffered four to five prior recurrences after previous surgeries performed without adjuvant radiotherapy and one patient who had a dedifferentiated chondrosarcoma had experienced gross tumor cut-through at the time of a prior surgical procedure at another institution. Patients who were treated with locally recurrent tumors after prior surgery were more likely to suffer another local recurrence, 4 of 14 versus 2 of 36 treated at the time of initial presentation, ρ = 0.013. Local control was the highest among the patients treated after gross total resection with negative margins (8 of 8) versus 36 of 42 among patients with positive margins or gross residual tumor, ρ = 0.028. Actuarial overall survival at 5 years was 90%.
Two of the locally recurrent patients with chondrosarcoma also developed distant metastases, as did four other patients whose tumors were locally controlled. Four patients died of progressive tumor and two patients died of unrelated causes (cardiac death, oral tongue cancer). One patient was lost to follow-up. Acute grade 3 toxicity consisted of acute pain from a sacral stress fracture after preoperative RT and surgery without late sequelae. Late grade 3 toxicity occurred in four patients. This included one neuropathy at 5.5 years (left foot drop, right lower extremity weakness, poor rectal tone, stress urinary incontinence) and one erectile dysfunction at 4 years unresponsive to sildenafil following 77.4 Gy (RBE) to unresected sacral chordomas, a sacral stress fracture following a fall 3 months after delivery of 77.4 Gy (RBE) which was managed with sacral nail fixation, and one rectal bleeding requiring transfusion following surgery and 70.4 Gy (RBE) of RT for a sacral chordoma.
This experience demonstrates that high-dose photon/ proton RT can be given to tumors involving the spine and paraspinal tissues. Morbidity to date appears to be acceptable. Although follow-up to date is still relatively short, encouraging results have been achieved with this treatment approach in a patient population with tumors that have historically been difficult to control.
Treatment Technique
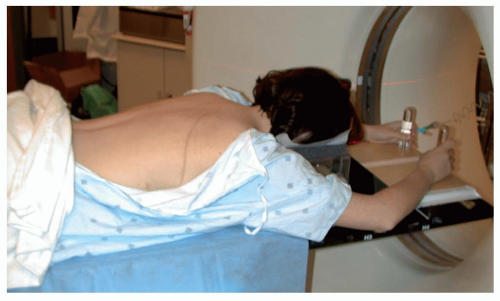
For patients with lesions of the thoracolumbosacral spine, initial evaluation includes an magnetic resonance imaging (MRI) scan of the lesion and computed tomography (CT) scan of the chest. For patients with tumors known to metastasize to bone (osteosarcoma, Ewing’s sarcoma), bone scan, positron emission tomography (PET) scan, or PET/CT are also appropriate. For the osteosarcoma and Ewing’s sarcoma patients, PET/CT as well as serum alkaline phosphatase and LDH have also been useful to assess response to induction chemotherapy. We have preferred to treat patients receiving protons in the prone position on the treatment couch. This minimizes the lateral penumbra of the proton beam by avoiding treatment through the couch. Patients are positioned with the head immobilized in a Duncan headrest and arms extended and holding posts (see Fig. 15.1). We had employed Alpha cradle immobilization in the past but it was at times difficult for these patients with spine tumors to climb into and out of these devices. Hence, we are currently using the less rigid immobilization
and relying on orthogonal pair diagnostic imaging on the treatment table before each treatment session for image guidance to treat these patients, by comparing spinal bony landmarks on these images with those from the digitally reconstructed radiographs (DRRs).
and relying on orthogonal pair diagnostic imaging on the treatment table before each treatment session for image guidance to treat these patients, by comparing spinal bony landmarks on these images with those from the digitally reconstructed radiographs (DRRs).
For patients with lesions involving vertebral levels abutting the spinal cord (above L3), treatment planning CT myelograms are performed to allow high precision localization of the spinal cord for organ at risk (OAR) avoidance. For patients who are treated postoperatively, surgical scars and drain sites are marked with wire in patients with tumors capable of seeding these scars (chordomas, high grade bone sarcomas including osteosarcoma, and MFH, and paravertebral soft tissue sarcomas) to allow their inclusion in the initial clinical target volume (CTV) that covers areas at risk for subclinical, microscopic tumor involvement. Patients are scanned using 2.5-3.75-mm CT slices with intravenous contrast. Oral contrast is utilized for lesions below the diaphragm, while iodinated esophageal paste is employed for lesions above the diaphragm. MRI scans are performed for image fusion for target localization.
The initial CTV encompasses the gross tumor with inclusion of adjacent tissues at risk for subclinical microscopic tumor involvement. This volume is treated to a dose of 50.4 Gy (RBE) at 1.8 Gy (RBE) per fraction for spindle cell sarcomas and 45 Gy (RBE) for Ewing’s sarcomas. This volume encompasses the entire involved vertebral body and at least the hemisacral segment for lateralized sacral lesions and the entire sacral segment for lesions involving both sides of the sacral segment. We have generally included one vertebral body above and below the involved levels as well as a 1-cm radial expansion into the adjacent soft tissues. There is no controlled data of which we are aware that addresses the optimum target volume for these patients. The volumes we have employed have been based on patterns of failure in surgical series. Our preferred approach has been to deliver preoperative RT to this initial CTV of 45 to 50.4 Gy (RBE) at 1.8 Gy (RBE) per day for lesions of the thoracic and lumbar spine followed by definitive surgery approximately 3 to 4 weeks later. Sacral lesions have a higher risk of delayed wound healing even in the absence of preoperative RT; therefore, for these lesions, reduced dose preoperative RT of 19.8 Gy (RBE) is delivered followed by surgery within 1 week of completion of radiation and the remainder of the dose to the initial CTV is delivered postoperatively beginning approximately 1 month after surgery. If postoperative radiation alone is employed, the initial CTV is designed to include surgical scars, drain sites, areas manipulated at the time of surgery, as well as spine stabilization hardware, with the exception of low grade chondrosarcomas, which rarely seed into these areas.
Surgical considerations and their impact on proton RT are extremely important for the management of these patients. In general, the optimal result of the surgical procedure at this location will be a gross total resection of tumor. Subtotal resections can be useful if they decompress the spinal cord, cauda, or sacral nerves without sacrifice of nerve function. Subtotal resections of tumors at the S1-3 levels are not advised if these are going to compromise sacral nerve root function responsible for urinary or rectal continence. Titanium rods are preferred over stainless steel to minimize artefacts and distortion on the treatment planning CT scans and to minimize the impact upon proton dosimetry. Transverse crosslinks between longitudinal rods that cross at the levels involved by tumor should be strongly discouraged as they make target identification extremely difficult on the treatment planning CT and MRI scans, introduce errors into the proton treatment planning algorithms, and alter the proton dose distribution in the vicinity of the tumor, producing shadowing cold spots behind the hardware and hot spots lateral to the hardware. Management of these patients by an experienced sarcoma team engaged in a treatment planning dialogue from the time of initial presentation is extremely important to optimize treatment outcome. For patients presenting with spinal cord compression, consideration should be given to initial posterior decompression if spine stability permits, to be followed by at least 19.8 Gy of radiation before more definitive resections through anterior or anterolateral approaches. This will minimize the risk of tumor seeding into surgically manipulated tissues. It will also markedly reduce the volume of tissue that will need to be irradiated, because stabilization hardware, surgical drain sites, surgical scars, and tissues manipulated at surgery will not need to be included in the radiation fields following preoperative RT, where the smaller fields described in the preceding text can be used.
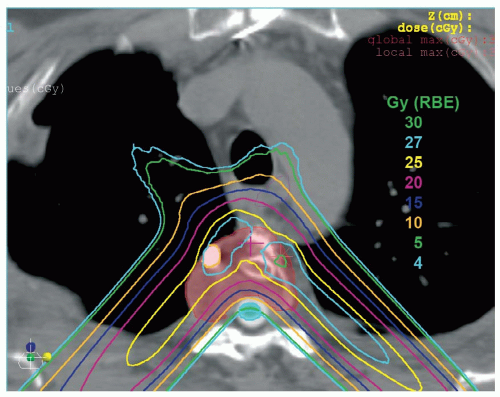
Another surgical consideration that is appropriate for many patients is intraoperative RT. One of the areas at highest risk for tumor involvement is the dura. It is often invaded by tumor on at least a microscopic and at times a gross basis. Resection of the dura poses the risk of contamination of the thecal space. Delivery of the desired dose to the dural surface, often in the range of 70.2 Gy (RBE), can be difficult to achieve even with protons (see Fig. 15.2). Hence orthovoltage intraoperative RT with 20-kV x-rays or a 90Y plaque might be important to help bring the dose to the dural surface to the desired dose.2
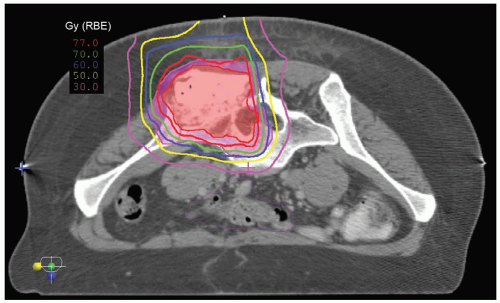
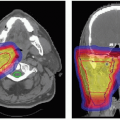
Boost radiation is delivered to spine sarcomas by shrinking field technique. The anatomic sites that were initially involved by gross tumor are boosted to a dose of approximately 70.2 Gy in the presence of microscopic residual disease and to approximately 77.4 Gy for gross residual disease. Whenever possible, contralateral nerve roots should be spared from the high-dose boost region (see Fig. 15.3).
The postoperative target definition can be very challenging for patients following sacral resections, as the sacrum, unlike the vertebral bodies, is not reconstructed. Hence, bowel and rectum often herniate into the space formerly occupied by the resected sacrum. The value of preoperative
RT is particularly evident for these patients. When preoperative RT is utilized, the bowel that falls into the resected sacral space does not need to be included in the target volume, which instead is focused on residual tumor in the remaining sacrum, gluteal, piriformis, and other muscles in apposition to tumor, sciatic notch region, and posterior pelvic tissues felt to be at risk for tumor involvement. Omental flaps or prosthetic tissue expanders may be helpful to exclude bowel in patients who will be undergoing radiotherapy in conjunction with sacral resections.
RT is particularly evident for these patients. When preoperative RT is utilized, the bowel that falls into the resected sacral space does not need to be included in the target volume, which instead is focused on residual tumor in the remaining sacrum, gluteal, piriformis, and other muscles in apposition to tumor, sciatic notch region, and posterior pelvic tissues felt to be at risk for tumor involvement. Omental flaps or prosthetic tissue expanders may be helpful to exclude bowel in patients who will be undergoing radiotherapy in conjunction with sacral resections.
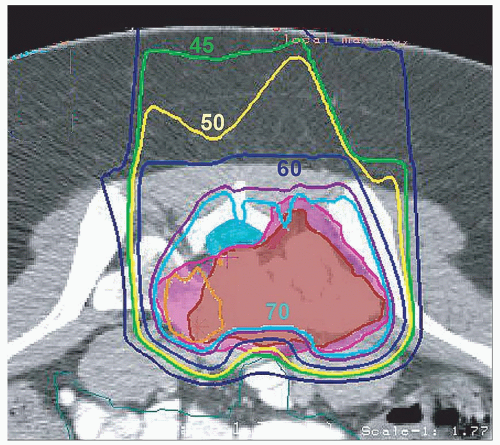
In patients with osteosarcomas, radiation has been started after several cycles of induction chemotherapy and our preference has been then to deliver radiotherapy concurrently with non-Adriamycin chemotherapy, often ifosfamide and etoposide (see Fig. 15.4). The dose to sites of microscopic residual disease for osteosarcomas following surgery is reduced slightly to approximately 66 Gy (RBE) and to gross residual disease following surgery or in unresected patients to approximately 70.2 Gy (RBE) because of the use of concurrent chemotherapy, although slightly higher doses might be considered in patients in whom the response to chemotherapy (as assessed by PET or PET/CT and alkaline phosphatase) is suboptimal. Ewing’s sarcomas are radiosensitive and are discussed in subsequent text. The initial CTV can be treated to 45 Gy (RBE) and then gross disease boosted to 55.8 to 59.4 further Gy (RBE) depending upon the bulk of the tumor. Radiotherapy is often initiated after approximately 12 weeks of chemotherapy and can be delivered concurrently with chemotherapy, generally ifosfamide/etoposide in our experience. Microscopic residual Ewing’s sarcoma after surgery is generally carried to a dose of 50.4 Gy (RBE). Giant cell tumors, in the setting of gross residual disease, are treated to a dose of 61.2 Gy (RBE).
An example of treatment planning for a carcinosarcoma of the spine is discussed in Chapter 9. Apertures have generally been designed to incorporate 3 mm for patient motion that might occur during the treatment session, based on studies of intrafraction motion performed on our patients;24 range compensation correction for motion is done by compensator smearing (see Chapter 8).
Normal Tissue Constraints
The normal tissue constraint for the spinal cord that we have employed for patients undergoing proton beam
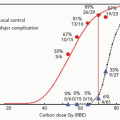
radiotherapy has been 63 Gy (RBE) to the cord surface and 54 Gy (RBE) to the cord center for a length of 5 cm of spinal cord. These are similar to doses that have been employed safely for patients treated to cervical spine lesions, where the surface of the cord has been limited to 67 Gy (RBE) and the center of the cord has been held to 53 Gy (RBE).25 No specific constraint has been employed in our protocols for the cauda equina and sacral nerves, but every attempt has been made to limit the dose to the structures, in particular to portions of the cauda not abutting the tumor and the contralateral sacral nerves (Fig. 15.3). Pieters et al. assessed the cauda equina tolerance in patients undergoing high dose photon/proton treatment.26 They noted that toxicity was seen in only approximately 5% of patients who received <65.8 Gy (RBE) at 2 Gy (RBE) per fraction (˜68.5 Gy [RBE] at 1.8 Gy [RBE] per fraction, using α/β ratio = 3). All LENT grade 4 neurologic toxicities occurred in patients who had received >73 Gy (RBE) to the cauda. More than half of the toxicities that they observed occurred 5 years or more after treatment. Caudal tolerance dose (defined as that dose expected to yield neurologic toxicity in 5% of patients at 5 years [TD 5/5]) appeared to be lower in men than women (TD 5/5 of 67 Gy [RBE] in women), for reasons that they were not able to determine.
radiotherapy has been 63 Gy (RBE) to the cord surface and 54 Gy (RBE) to the cord center for a length of 5 cm of spinal cord. These are similar to doses that have been employed safely for patients treated to cervical spine lesions, where the surface of the cord has been limited to 67 Gy (RBE) and the center of the cord has been held to 53 Gy (RBE).25 No specific constraint has been employed in our protocols for the cauda equina and sacral nerves, but every attempt has been made to limit the dose to the structures, in particular to portions of the cauda not abutting the tumor and the contralateral sacral nerves (Fig. 15.3). Pieters et al. assessed the cauda equina tolerance in patients undergoing high dose photon/proton treatment.26 They noted that toxicity was seen in only approximately 5% of patients who received <65.8 Gy (RBE) at 2 Gy (RBE) per fraction (˜68.5 Gy [RBE] at 1.8 Gy [RBE] per fraction, using α/β ratio = 3). All LENT grade 4 neurologic toxicities occurred in patients who had received >73 Gy (RBE) to the cauda. More than half of the toxicities that they observed occurred 5 years or more after treatment. Caudal tolerance dose (defined as that dose expected to yield neurologic toxicity in 5% of patients at 5 years [TD 5/5]) appeared to be lower in men than women (TD 5/5 of 67 Gy [RBE] in women), for reasons that they were not able to determine.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree