Author
Patients/lesions
Camera
Results
Horger [41]
47/104
GE Millenium 1 slice
Decrease of unclear lesions, 15 % indeterminate findings
Römer [42]
44/52
Siemens Symbia T2/2 slices 40 mAs
Decrease of unclear lesions to 8 %
Utsunomya [48]
45/82
GE/8 slices – 140 mAs
n.a.
Strobel [49]
37/42
GE 64 slices – 100–700 mAs
Decrease of unclear lesions, external fusion!
Ndlovu [44]
42/189
GE Infinia Hawkeye 1 slice, 2.5 mAs
Decrease of unclear lesions to 14 %
Helyar [43]
40/50
Philips 16 slices 100 mAs
Decrease of unclear lesions to 18 %
Zhao [50]
125/141
Philips 6 slices 140 mAs
Decrease of unclear lesions to 4 %
Palmedo [46]
308/839
GE Infinia Hawkeye 4 slices, 2.5 mAs
Whole-body SPECT/CT, per-patient analysis, 32.1 % downstaging
We investigated the impact of additional SPECT/CT with low-dose application and mainly whole-body technique in 451 cancer patients. Most of the patients (76.3 %) were examined because of breast or prostate cancer. For the remaining 23.7 % of the study population, the cause of examination was other primary tumor (lung cancer, thyroid cancer, gastric cancer, primary bone cancer).
Whole-body scintigraphy (WBS), SPECT, and SPECT/CT were performed in all patients for staging and restaging. This means that also patients with normal whole-body imaging underwent SPECT and SPECT/CT. SPECT/CT of the body stem was performed in 81 % of patients. All data were acquired with a combined SPECT/CT in-line system using a multislice helical CT scanner and allowing the acquisition of co-registered CT and SPECT images in one session.
In the group of 406 evaluable patients, clinical follow-up was used as the gold standard (every 3–6 months during 1 year after imaging). For follow-up, clinical examinations, medical reports, imaging results, and tumor markers were used. A multistep analysis per lesion as well as per patient was performed [45]. The evaluation of images was performed by a central consensus reading [46]. Clinical relevance was expressed by means of down- and upstaging rates on a per-patient basis.
Bone metastases were confirmed and excluded in 102 and 304 cases, respectively. We have examined a total of 1,134 lesions. Most lesions were located in the spine and pelvis. However, a significant number of lesions were found in other parts of the skeleton (Table 7.2). Scoring by whole-body scintigraphy, additional SPECT, and additional SPECT/CT led to indeterminate lesions in 19.9, 21.3, and 3.7 %, respectively. Astonishingly, the rate of unclear findings was similar for whole-body and SPECT imaging. It can be supposed that the number of unclear SPECT lesions is higher when also regions with normal planar whole-body images are investigated by SPECT. This is more often the case when SPECT of the body stem is performed.
Table 7.2
Distribution of bone lesions
Anatomical region | Number of lesions | Percent |
|---|---|---|
Cervical spine | 45 | 4.0 % |
Thoracic spine | 274 | 24.2 % |
Lumbar spine | 287 | 25.3 % |
Pelvis | 256 | 22.6 % |
Ribs | 105 | 9.3 % |
Sternum | 24 | 2.1 % |
Shoulder | 89 | 7.8 % |
Cranium | 13 | 1.1 % |
Femora | 27 | 2.4 % |
Trunk | 14 | 1.2 % |
Total | 1,134 | 100 % |
With regard to the patient-based analysis, we found the following results: in the total patient group, sensitivities, specificities, and negative and positive predictive values on a per-patient basis for WBS, SPECT, and SPECT/CT were, respectively, 94, 78, 97, and 59 %; 94, 71, 97, and 53 %; and 96, 95, 99, and 87 %. In all subgroups, specificity and positive predictive value were significantly (p < 0.01) better using SPECT/CT. Downstaging of metastatic disease in the total, breast cancer and prostate cancer groups using SPECT/CT was possible in 32.5, 33.8, and 29.5 % of patients, respectively. Upstaging of previously negative patients by additional SPECT/CT was observed in 2.2 % (3 cases) of breast cancer patients. Further diagnostic imaging procedures for unclear scintigraphic findings were necessary in only 2 % of the patients. SPECT/CT improved diagnostic accuracy for defining the extent of multifocal metastatic disease in 32 % of these cases.
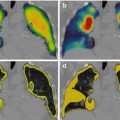
In our study, planar whole-body bone scintigraphy showed a sensitivity of 94 % and specificity of 78 % in the total patient group. These values are within the range reported in previous studies, demonstrating a sensitivity and specificity of 80–95 % and 62–81 %, respectively [33, 34]. We could demonstrate that specificity can be improved significantly by adding SPECT/CT to whole-body scintigraphy. On a per-patient analysis, specificity was increased from 78 to 95 % by addition of SPECT/CT. For 163 patients showing unclear or suspicious findings on whole-body scintigraphy, SPECT/CT could truly identify benign bone disease in 53 cases (Fig. 7.1a–c). Thus, about one third (32.5 %) of the patients suspected to have bone metastases or unclear findings could be converted into metastasis-free patients. If whole-body scintigraphy together with SPECT were considered, the conversion rate was even higher at 39.5 % because SPECT generated more unclear lesions than whole-body scintigraphy alone. These findings differ from previous studies and might be explained by our different study design. We performed mainly multi-field SPECT and SPECT/CT (mainly of the body stem) as a general procedure in all patients. This means that not only suspicious findings on planar whole-body scintigraphy were reevaluated by SPECT and SPECT/CT (as done in the cited studies) but also normal planar images. This resulted in many positive SPECT and SPECT/CT findings that were negative on planar images. The interpretation of this negative-positive image constellation seems to cause more difficulty for SPECT alone to differentiate between benign and malignant lesions.
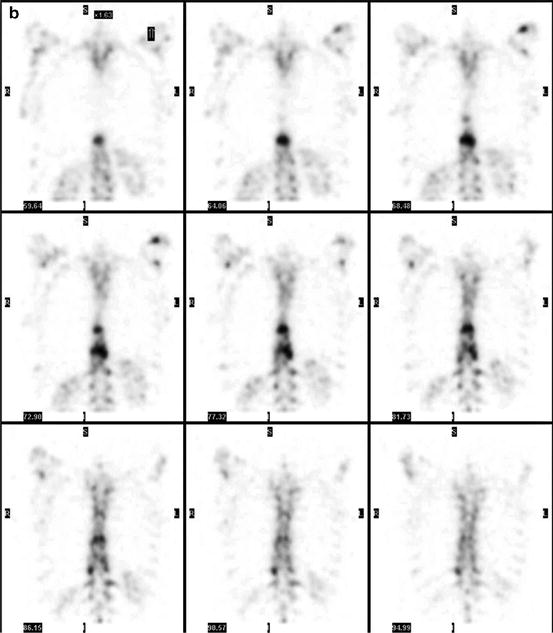
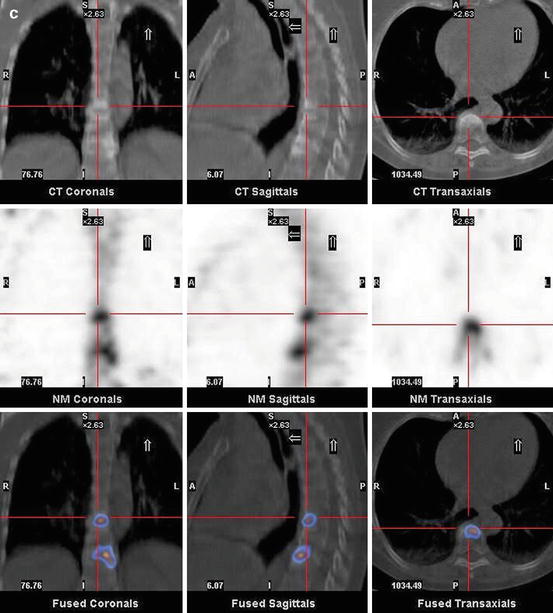



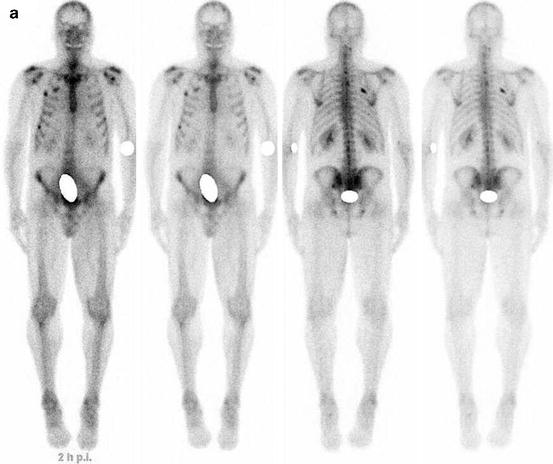
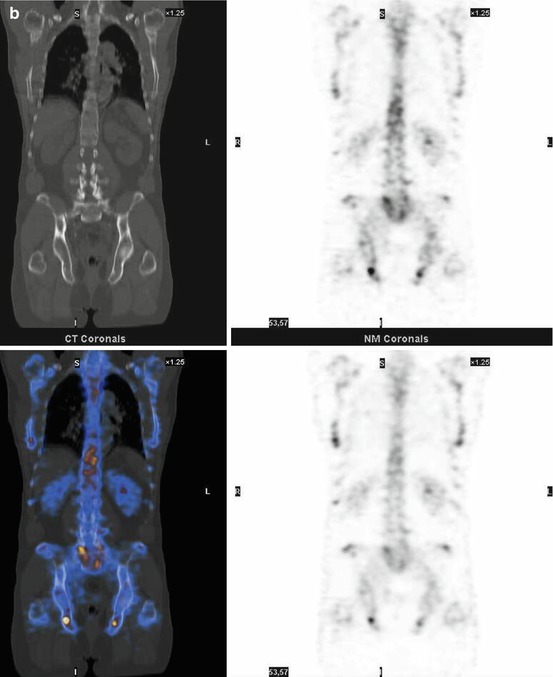
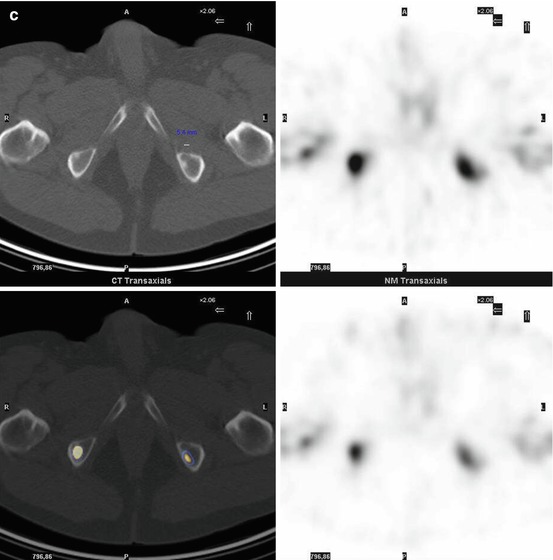
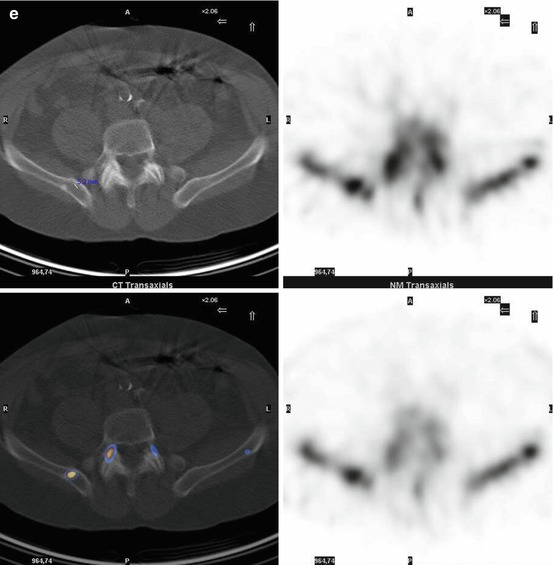
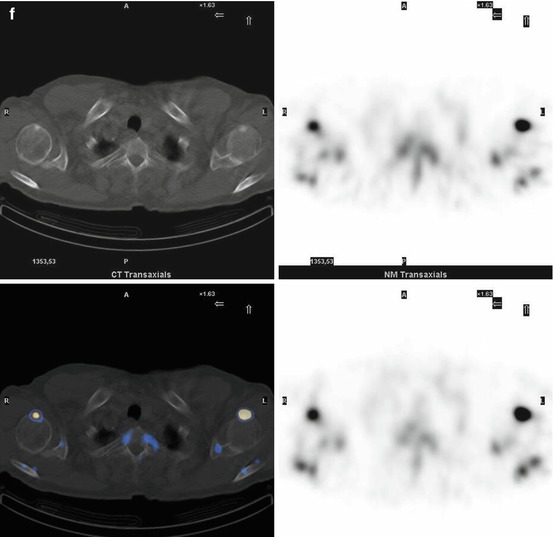
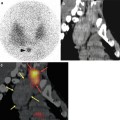
Fig. 7.1
A 67-year-old female patient with newly diagnosed breast cancer and back pain in the thoracic region. Planar whole-body scintigraphy shows two suspicious accumulations in the thoracic column (a) which are also confirmed by SPECT (b). The lesions were localized at thoracic vertebras 7 and 9. SPECT/CT identifies these lesions as degenerative changes at thoracic vertebras 7/8 and 9/10 (c). Due to this SPECT/CT finding, the patient was downstaged correctly to nonmetastatic disease. This therapeutically relevant imaging result was confirmed by further follow-up of the patient
Our data show that sensitivity also can be improved by SPECT/CT. However, this improvement was only the case in breast cancer patients, resulting in an increase of sensitivity from 90.0 to 97.7 % because three primarily negative patients were converted into metastatic-positive patients.
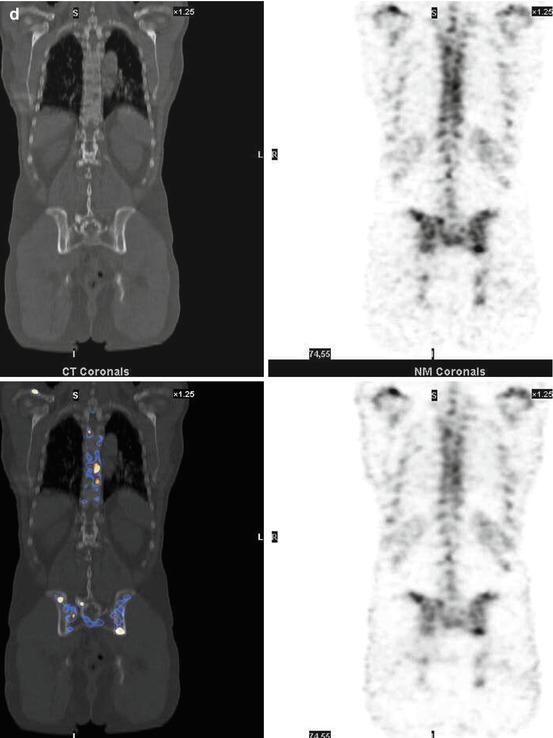
One important aspect of our study was evaluation of the clinical relevance of SPECT/CT. We estimate that our patient population in this clinical setting represents rather the situation of a clinical everyday practice and not that of a specially selected patient group. Furthermore, we performed a follow-up of patients to meet a gold standard for diagnostic accuracy of SPECT/CT. The number of patients who needed additional diagnostic imaging like x-ray, CT, or MRI to characterize unclear findings was significantly reduced from 29.3 % with whole-body scintigraphy to 2.1 % with SPECT/CT. One third of patients with suspicious or unclear findings were truly downstaged to nonmetastatic benign disease. This effect was observed equally in all three tumor groups (breast cancer, prostate cancer, lung and other cancers) and is of high therapeutic relevance. In addition, the upstaging rate of 2.2 % in (whole-body) scintigraphically negative breast cancer patients is clinically important. In 32 % of patients with metastatic disease, the extent of metastases was more precisely defined by SPECT/CT, indicating an improvement in planning further patient treatment (Figs. 7.2 and 7.3).