of head trauma and assess blood flow in cases of cerebrovascular disease. A sonographic finding or suspected lesion can be further characterized with computed tomography (CT) or magnetic resonance imaging (MRI) when the patient is more stable.
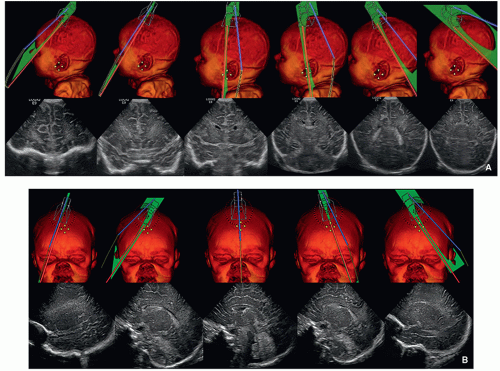 FIGURE 2.1 A: Positions of the transducer for coronal ultrasound images through the brain. B: Positions of the transducer for sagittal ultrasound images through the brain. |
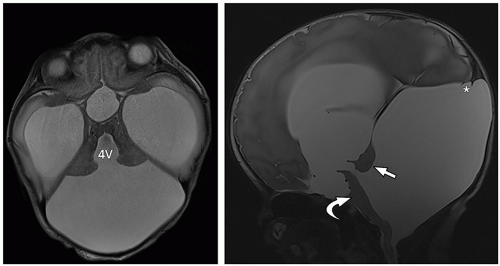
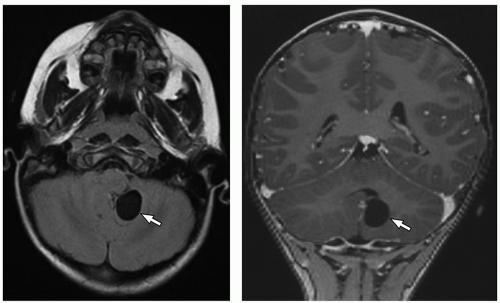
tectum of the midbrain, and fourth ventricle can be identified on this midline image.
factors necessitate use of sedation or general anesthesia in a proportion of young pediatric patients. Various techniques have been used to reduce the need for sedation, including mock MRI, which acclimatizes the child to the MRI scanner, and technologies that decrease acquisition times, such as multichannel head coils, parallel imaging, and motion compensation techniques. In order to ensure that the information required to answer the clinical question is acquired in the shortest possible time before lack of patient cooperation becomes problematic, it is important to acquire the most important sequences at the start of the examination and actively monitor the MRI exam in a pediatric patient.
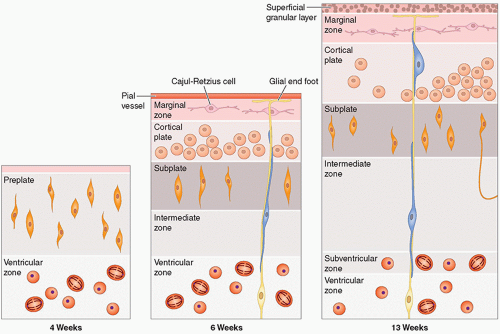
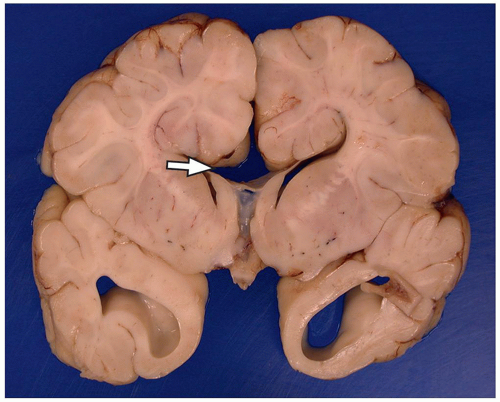
they differentiate to form the normal six-layered cortex (Fig. 2.4).
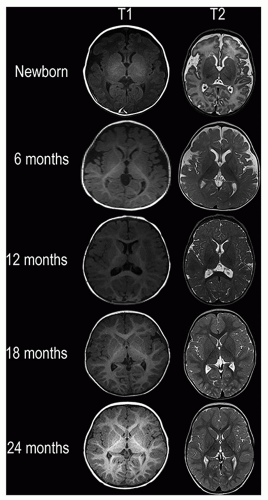
are affected by a number of factors, including changes in the structure and chemical composition of the axon and the surrounding myelin and the degree of axonal ensheathment by myelin. Most authors believe that signal changes on T1-weighted MR images correspond with the increase in the certain lipids occurring during myelin formation. The changes on T2-weighted MR images can be explained by myelin sheath maturation and decrease in the water content of the white matter indicated in vitro by thickening and tightening of the spiral of myelin around the axon.
TABLE 2.1 Milestones of Myelination | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
TABLE 2.2 Classification of Congenital Brain Malformations | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
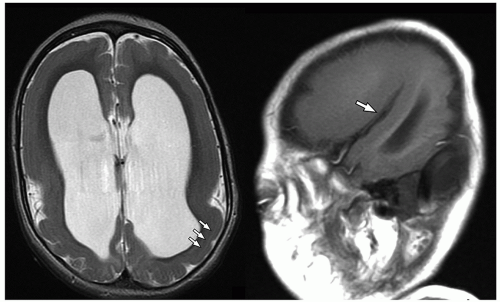
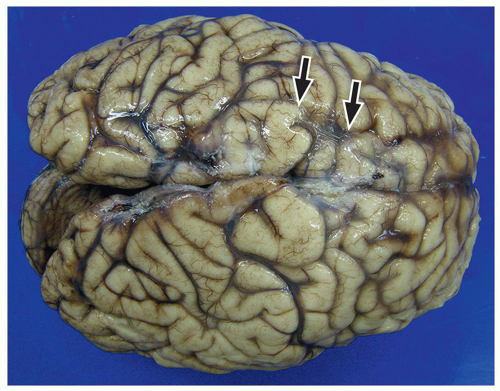
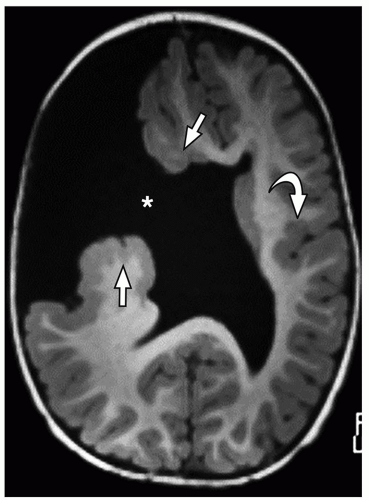
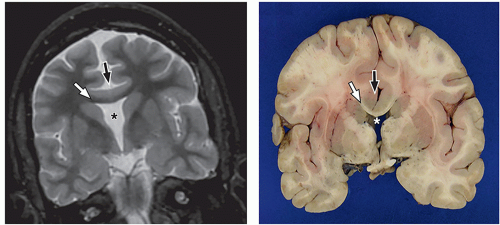
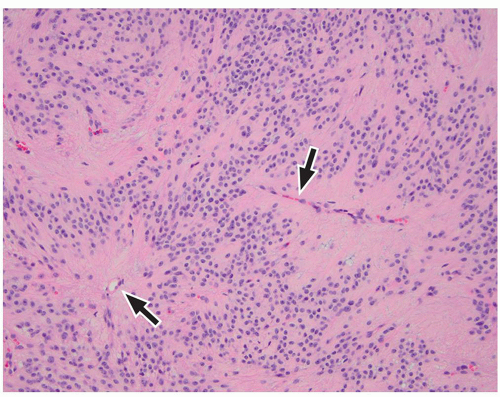
lamination of the cortex. The overfolding is usually microscopic, and the abnormal lamination either unlayered or four-layered in most described cases. Polymicrogyria is most commonly seen in the perisylvian cortex, but almost all cortical regions can be involved. Polymicrogyria is a highly heterogeneous disorder in terms of its pathogenesis, distribution, pathologic appearance, and clinical and imaging features. The clinical presentation is varied, and patients present at all ages from the neonatal period until late adulthood.
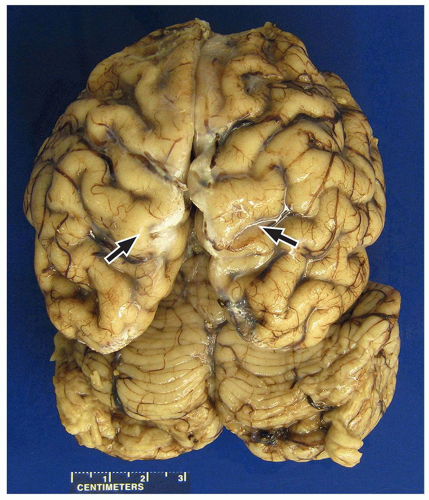 FIGURE 2.7 Pachygyria in an 11-year-old girl with microcephaly. The cerebral cortex shows pachygyria, characterized by a paucity of gyri (black arrows). |
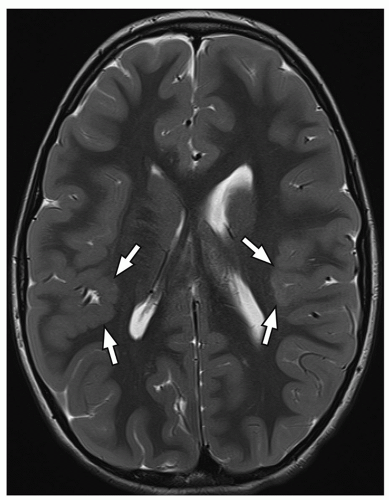
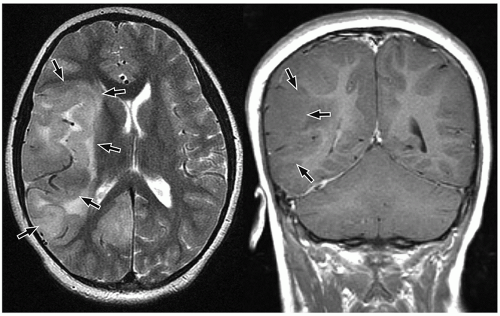
On MRI, polymicrogyria is characterized by an irregular cortical surface and “stippled” gray-white junction with regions of apparent cortical thickening (Figs. 2.8 and 2.9).
ependyma to the pia. The overlying cortex is thin, and the underlying ventricle often appears distorted. These foci follow gray matter on all sequences, do not demonstrate edema, and do not enhance, which differentiates them from glioneuronal tumors like gangliocytomas.
the abnormalities may also involve eloquent areas, and resection may not be an option in these cases. Therefore, other diagnostic imaging techniques such as FDG-PET, magnetoencephalography (MEG), DTI, and intracranial electroencephalography (EEG) are widely used to establish the diagnosis and plan management.
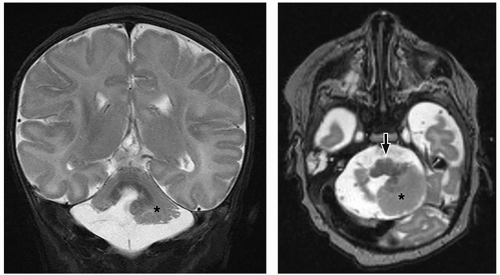
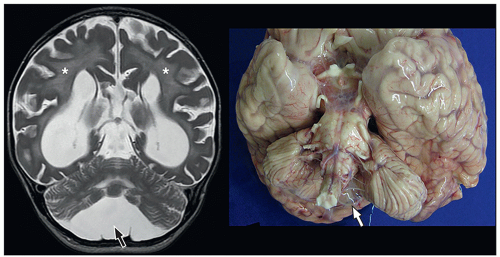
cerebellum (i.e., cerebellar hypoplasia). In the severe variant, both hemispheres and vermis are almost completely absent. The brainstem, particularly the pons, is hypoplastic.
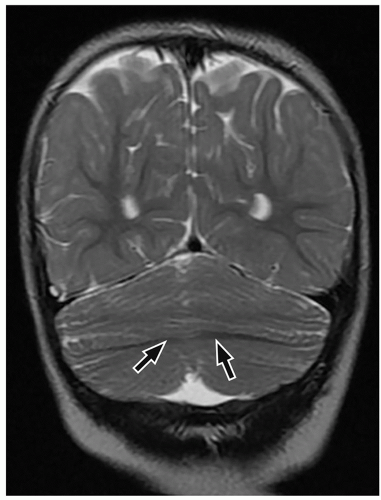
to the folia and is usually associated with hypoplasia of the pons. In contrast, cerebellar atrophy refers to a small cerebellum with prominent cerebellar fissures or evidence of progressive volume loss shown on serial imaging.
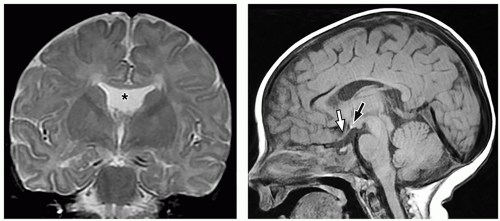
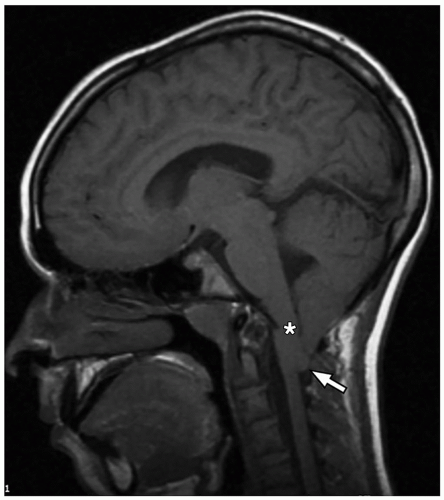
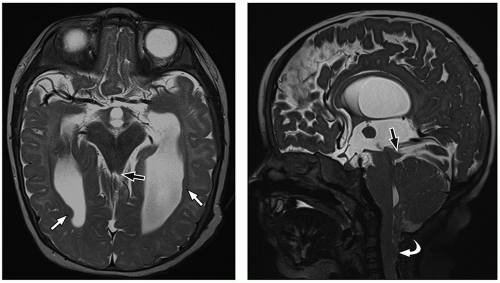
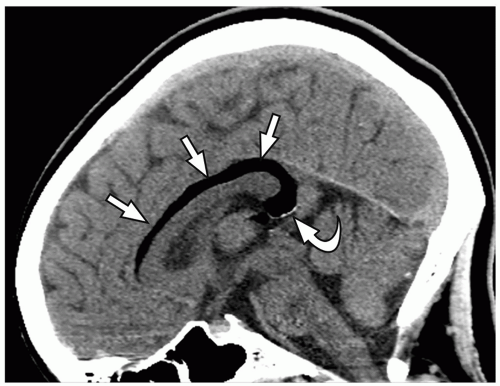
thalami, fornices, and tectum may be present. Other midline and forebrain anomalies including absent cavum septum pellucidum, absent olfactory bulbs, and corpus callosum dysgenesis can be also seen.
urgent evaluation. Vocal cord abduction, paresis, or paralysis resulting from dysfunction of the vagus nerve causes the inspiratory stridor. Cranial nerve dysfunction is attributed to various causes including caudal traction on the nerve by the herniated medulla/medullary kink, lower brainstem compression, or an abnormally formed dorsal motor nucleus within the brainstem.
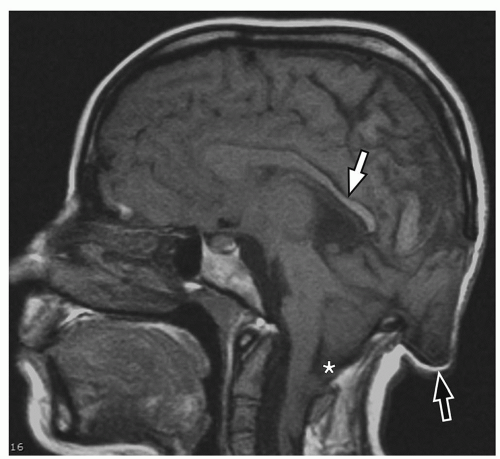
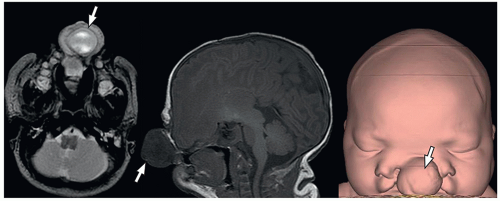
containing only CSF), and atretic cephaloceles (containing dura, fibrous tissue, and degenerated brain tissue).
Arachnoid cysts and neuroepithelial cysts are discussed in the following section. The information regarding leptomeningeal cysts is included in Chapter 1 of this book.
TABLE 2.3 Other Congenital TORCH Infections | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
prevent fetal transmission of the CMV virus, but do play a role in reducing severity of disease. Most infants have silent infections following recurrent rather than primary maternal infection. CMV is considered the leading infectious cause of sensorineural hearing loss in the post-rubella vaccination era. Sensorineural hearing loss occurs in around 10% of infected neonates.30 Additional clinical manifestations in affected infants include microcephaly, chorioretinitis, and seizures.
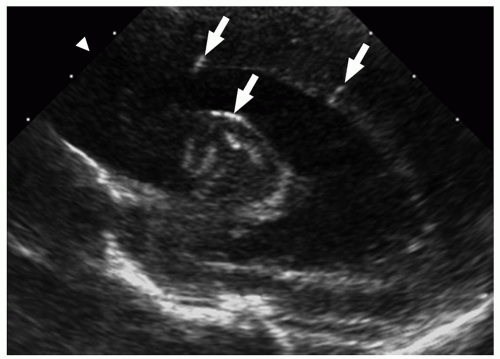 FIGURE 2.29 Congenital cytomegalovirus infection in a 2-day-old boy. Parasagittal ultrasound image shows areas of increased echogenicity consistent with periventricular calcifications (arrows). |
puncture, it is important to recognize that normal CT findings may not be sufficient to indicate normal intracranial pressure in pediatric patients with bacterial meningitis. Review of the literature indicates that herniation is unlikely in children with bacterial meningitis unless they have focal neurologic findings or coma.32 In addition, the results of an imaging study do not exclude or prove the presence of acute meningitis. Diagnosis should therefore be made based on clinical history, examination findings, and results of laboratory tests.
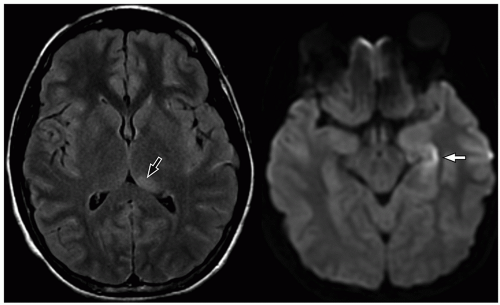
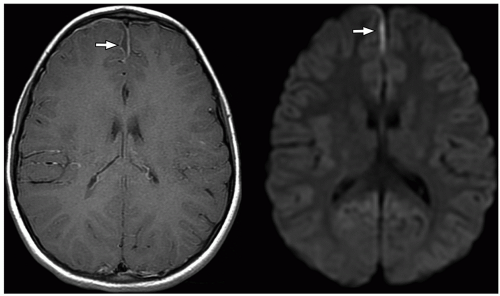
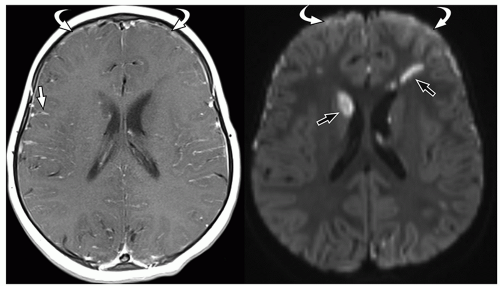
more commonly than adults. Organisms from these foci are released into the subarachnoid space causing meningitis. Meningitis is typically most severe in the basal cisterns and leads to secondary complications, including multiple cranial nerve palsies, vasculitis of the lenticulostriate and thalamoperforating vessels resulting in secondary infarction of the basal ganglia, and hydrocephalus secondary to blockage of the fourth ventricular outlet foramina. It is important to note that hydrocephalus in patients with tuberculous meningitis may need to be treated surgically. This can be determined with an emergent CT at presentation, followed by MRI later.34 Imaging using diffusion-weighted MRI is important to document the presence of infarcts, which correlates with poor outcome. In particular, bilateral basal ganglia infarcts have a poor prognosis. Border zone necrosis seen in these patients needs to be differentiated from necrosis in older children with herpes, which is most often seen at the insular cortex.35
perimesencephalic cisterns, suprasellar cisterns, and sulci over the convexities are commonly affected.
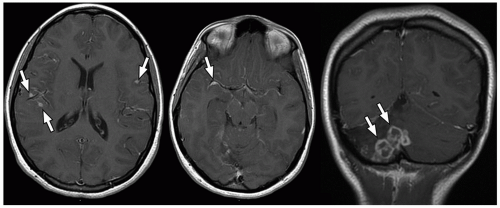
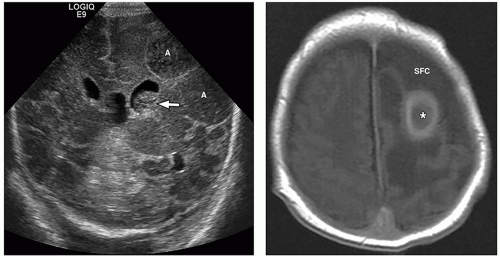
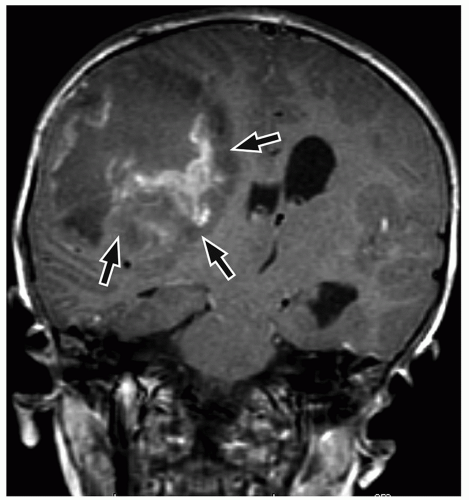
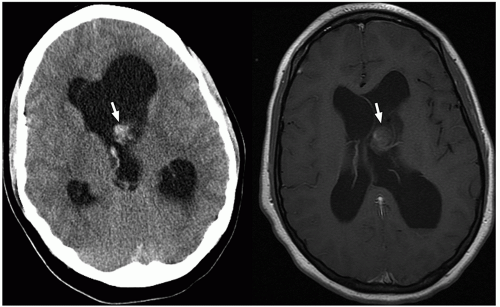
demonstrate enhancement of the walls of the lesion following gadolinium-based contrast administration (Fig. 2.35). Diffusion-weighted MR imaging is helpful to differentiate between an abscess and a necrotic tumor. Therefore, it must be performed in all cases of suspected CNS infection because almost all pyogenic abscesses demonstrate decreased apparent diffusion coefficient (ADC) values, indicating decreased water diffusion compared with nonpyogenic lesions.
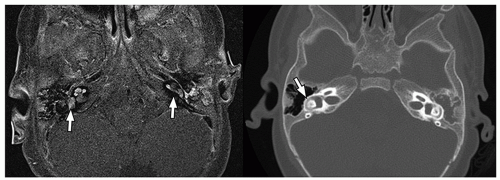
detect early fibrosis within the inner ear structures and labyrinthitis ossificans33 (Fig. 2.38).
tumors in childhood is ˜4.5 cases per 100,000 person-years.42 In young children <3 years of age, supratentorial tumors are more common than infratentorial tumors.42 In children between 4 years and 10 years of age, infratentorial tumors occur more frequently. Supra- and infratentorial tumors occur equally after 10 years old age.42
TABLE 2.4 Clinical Manifestations and Central Nervous System Imaging Features of Fungal Infections | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
TABLE 2.5 Common Anatomic Locations of Pediatric Brain Tumors | |||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||
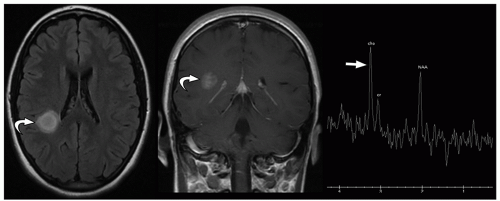
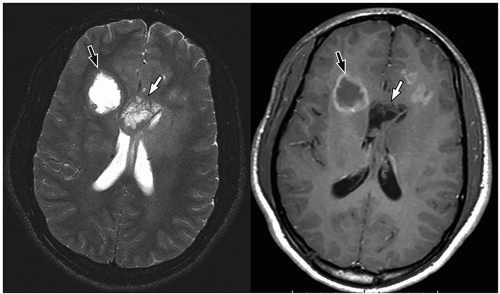
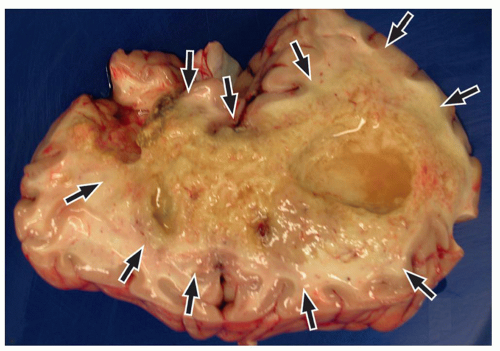
be seen (Fig. 2.42). Intratumoral bleeding is also common because of the abnormal, rich vasculature that characterizes these tumors. Histologically, glioblastoma multiforme consists of poorly differentiated glial cells, often with pronounced variation in nuclear size and shape (anaplasia or pleomorphism).
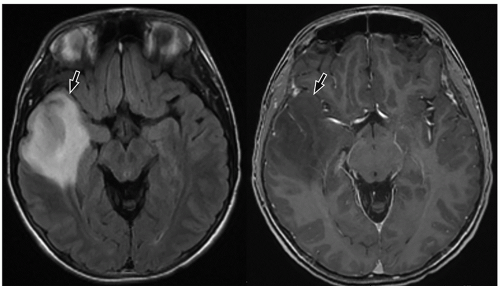
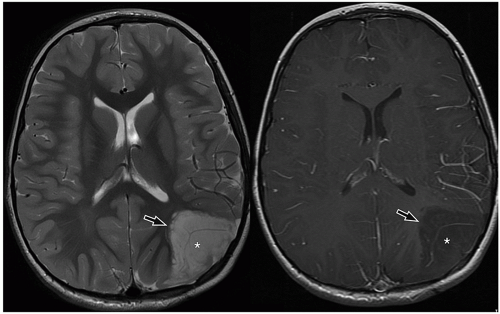
slow-growing peripherally located lesions, remodeling of the inner table of the skull is a common finding.
in young adults. Both tumors arise in the cerebral cortex, most commonly in the temporal lobe. Presenting symptoms depend upon the size and location of tumors and usually include seizures. In particular, complex partial seizures are commonly associated with temporal lobe tumors.
of enhancement may suggest the diagnosis of these tumors, although the appearance is still nonspecific.
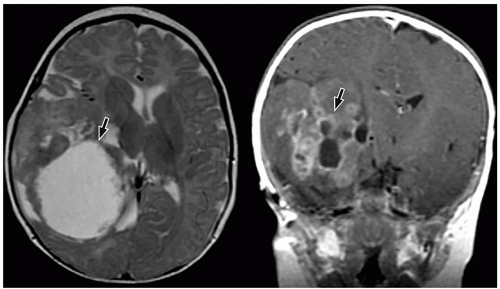
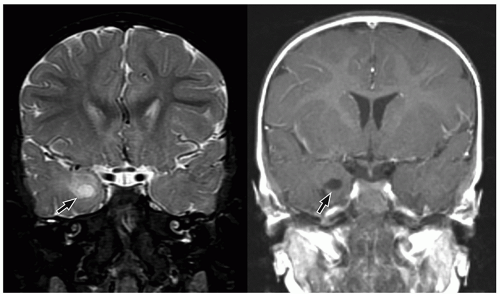
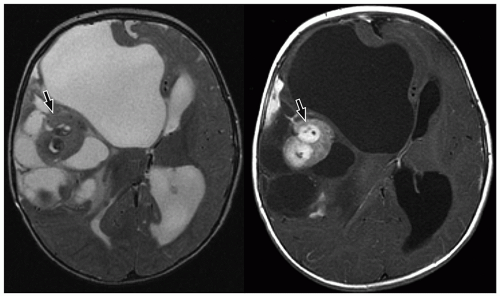
CNS tumors in childhood.42 DNETs are located most commonly in the temporal and parietal lobes. Most lesions arise from the cortical gray matter, and associated cortical dysplasia has been reported in more than 80% cases.51,52
bilateral optic nerve tumors are virtually pathognomonic of this diagnosis. Optic pathway gliomas may involve the optic nerves, optic chiasm, optic tract, lateral geniculate bodies, and/or optic radiations. Optic gliomas occur with increasing frequency in patients with NF1 (20% to 50%).56 On the other hand, up to 24% of patients with NF1 have optic pathway gliomas.57 Tumors in children with NF1 are reportedly less aggressive than those in children without NF1 and tend to be bilateral.
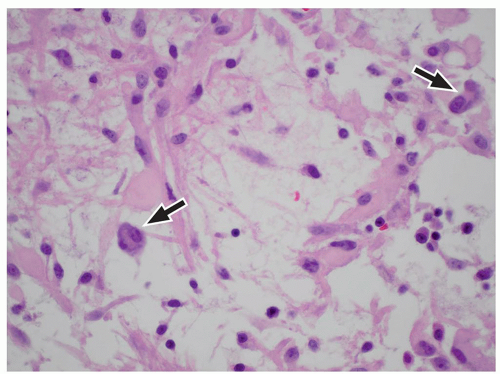
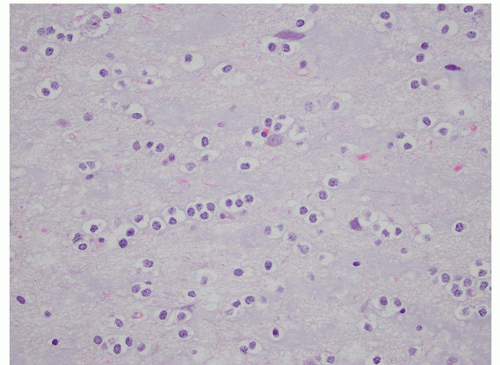
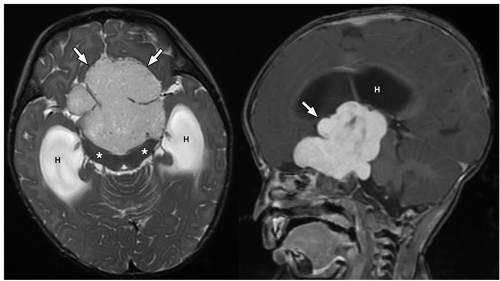
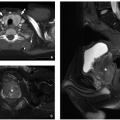
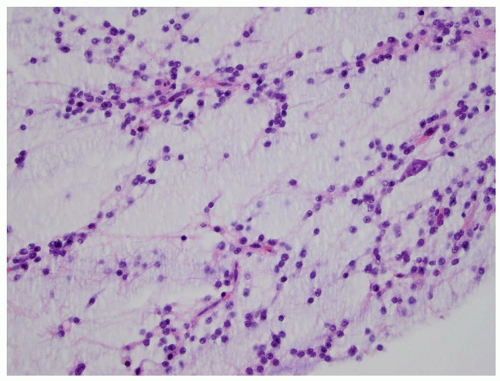 FIGURE 2.54 Neurocytoma. This intraventricular mass from the same patient (Figure 2.53) shows round uniform cells interspersed with “nucleus-free” zones of fibrillary neuropil (hematoxylin and eosin, original magnification, 400×). |
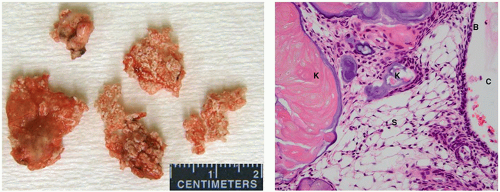
The adamantinomatous type is more common in children, whereas the squamous-papillary variant tends to occur in adults.59,60 Although they are benign, they can invade surrounding structures in the sellar and parasellar regions, eliciting a gliotic response and making resection challenging.
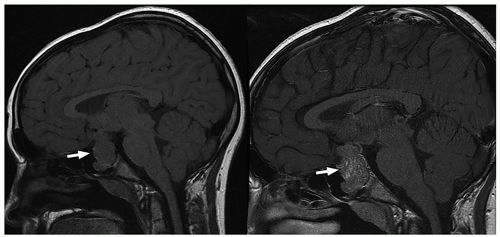
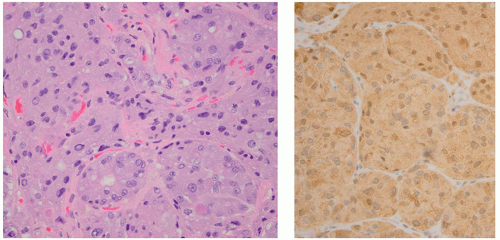
ng/mL.65 Functioning microadenomas are usually treated with medical therapy, although some surgeons prefer upfront operative management in pediatric patients. Microscopically, pituitary adenomas may be classified based on their architectural pattern and hormonal content as assessed by immunohistochemical staining (Fig. 2.59).
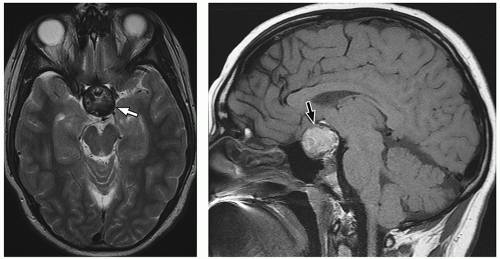
MR images. Intracystic nodules have also been described, but may be difficult to visualize because of similar signal intensity of the cyst fluid and the nodule. Although imaging findings may be helpful for differentiating these lesions from other sellar or suprasellar lesions, radiologic findings can be nonspecific, and features can overlap with cystic craniopharyngiomas or cystic pituitary adenomas. Follow-up imaging is required in many cases, and occasionally, cyst aspiration may be required to establish a diagnosis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree