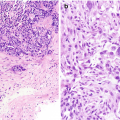
Fibrocystic changes . (a) Left mediolateral oblique (MLO) view shows numerous scattered oval circumscribed masses. (b, c) Ultrasound images of the left breast demonstrate corresponding cysts and clusters of cysts. (d) Ultrasound image of the contralateral right breast also demonstrates a cluster of cysts consistent with fibrocystic change
Fine Needle Aspiration (FNA)
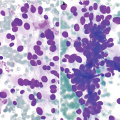
Aspirates can show variable cytomorphology, depending on the proportion of fibrous tissue to cystic spaces.
Cyst contents: Characterized by macrophages, inflammatory cells, and proteinaceous material (Fig. 11.2a).
Adipose tissue (Fig. 11.2b).
Clusters of small ductal cells and apocrine metaplastic cells.
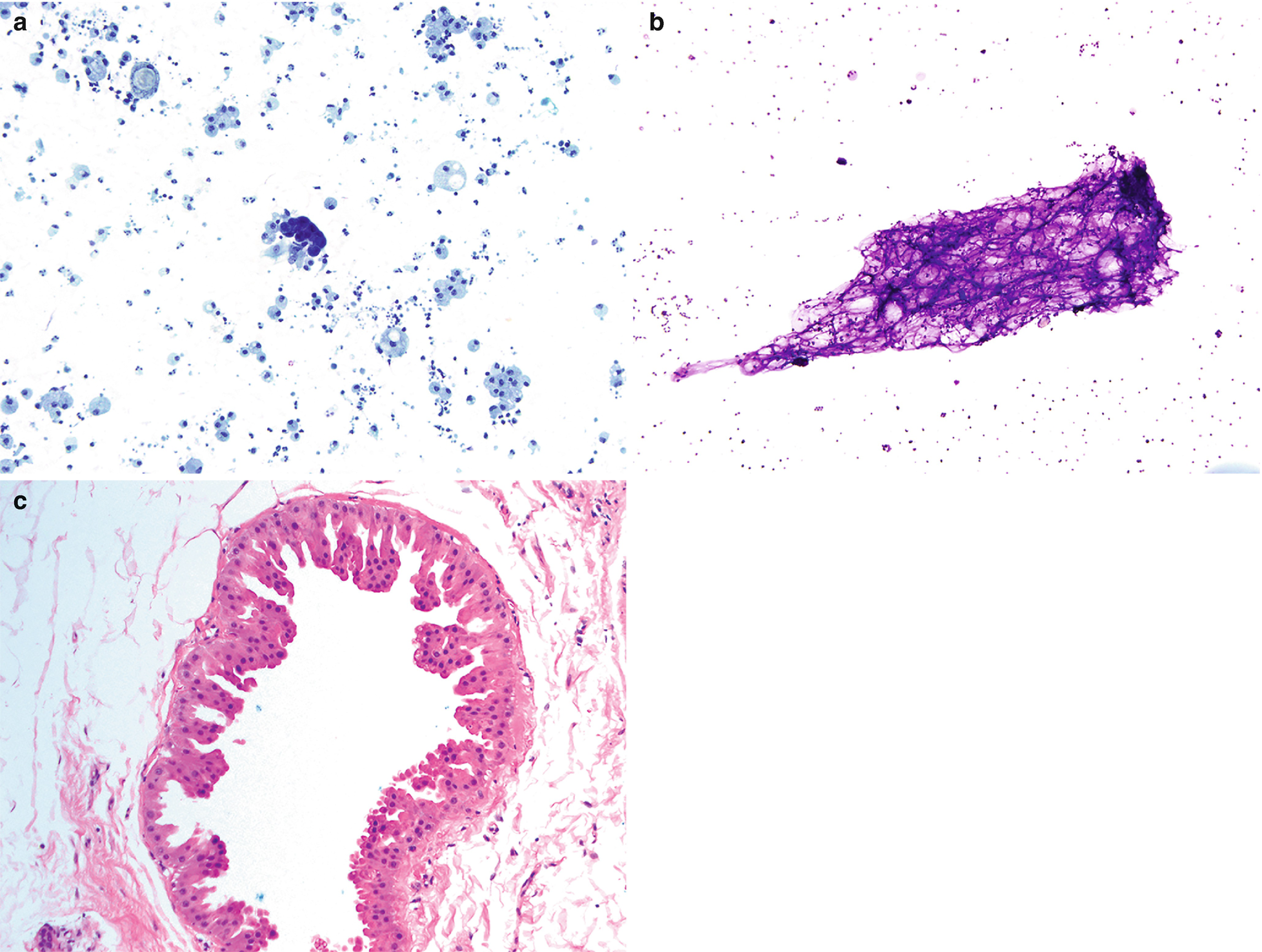
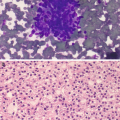
Fibrocystic changes . (a) Aspirates can be quite variable, depending on the proportion of fibrous tissue to cystic spaces. This aspirate is predominantly characterized by cyst contents with numerous macrophages with abundant foamy cytoplasm and ovoid to kidney bean-shaped nuclei. Scattered inflammatory cells are usually also present; few neutrophils and lymphocytes are seen (Papanicolaou stain, 200×). (b) Scattered clusters of mature adipose tissue can also be seen in aspirates of fibrocystic changes, as displayed in this case (DiffQuik stain, 100). (c) Fibrocystic changes. Cyst with apocrine metaplasia. Dilated cyst lined by epithelial cells with round nuclei, variably prominent nucleoli, and abundant eosinophilic cytoplasm with apical snouting and supranuclear eosinophilic granules (hematoxylin and eosin stain [H&E], 200×)
Core Biopsy
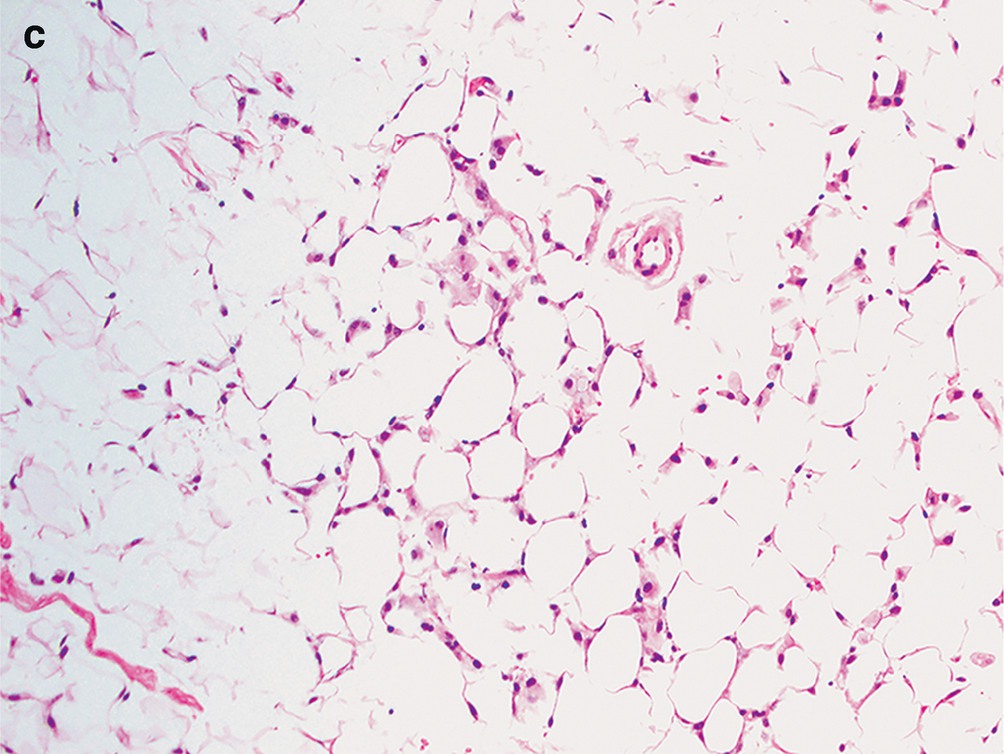
Dilated cysts lined by apocrine metaplastic cells with abundant granular eosinophilic cytoplasm, round nuclei, and prominent nucleoli. Intervening fibrotic stroma and adipose tissue (Fig. 11.2c).
Fat Necrosis
Clinical
May mimic carcinoma both clinically and mammographically as a mass-forming lesion, typically in patients following trauma or previous surgery.
Can also be seen in male patients [3].
Radiology
Mammography
Ultrasound
Variable appearance from oval circumscribed hypoechoic mass to irregular hypoechoic mass with angular margins and echogenic rim (Fig. 11.4).
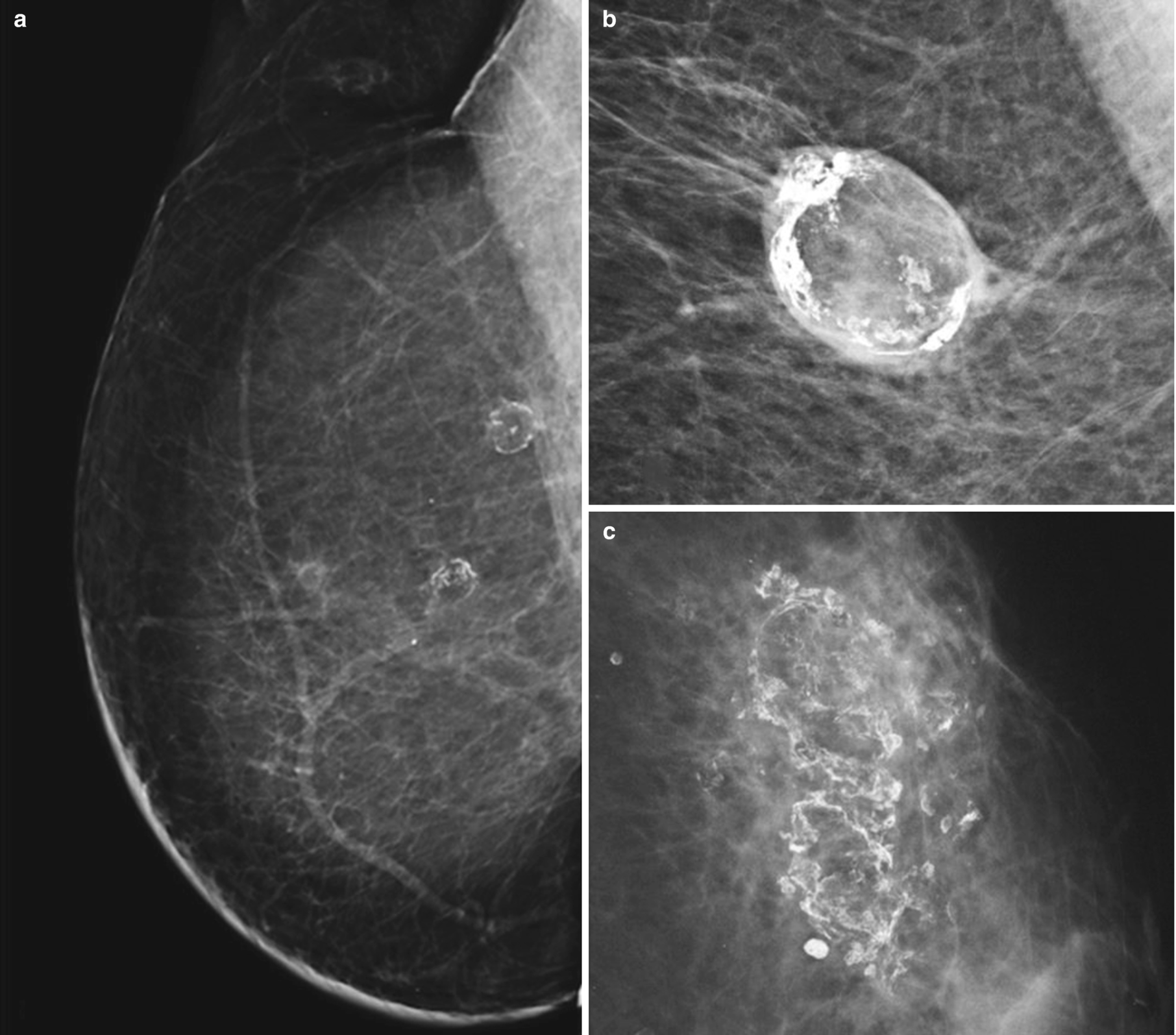
Fat necrosis . (a) Right MLO digital mammogram image from patient with history of mastectomy and transverse rectus abdominis muscle (TRAM) flap reconstruction shows rim-calcified lucent masses in the upper breast consistent with fat necrosis. (b) Spot magnification view demonstrates an oval, circumscribed, rim-calcified mass consistent with an oil cyst from fat necrosis. (c) Left MLO digital mammogram demonstrates coarse dystrophic calcifications with associated lucency characteristic of fat necrosis
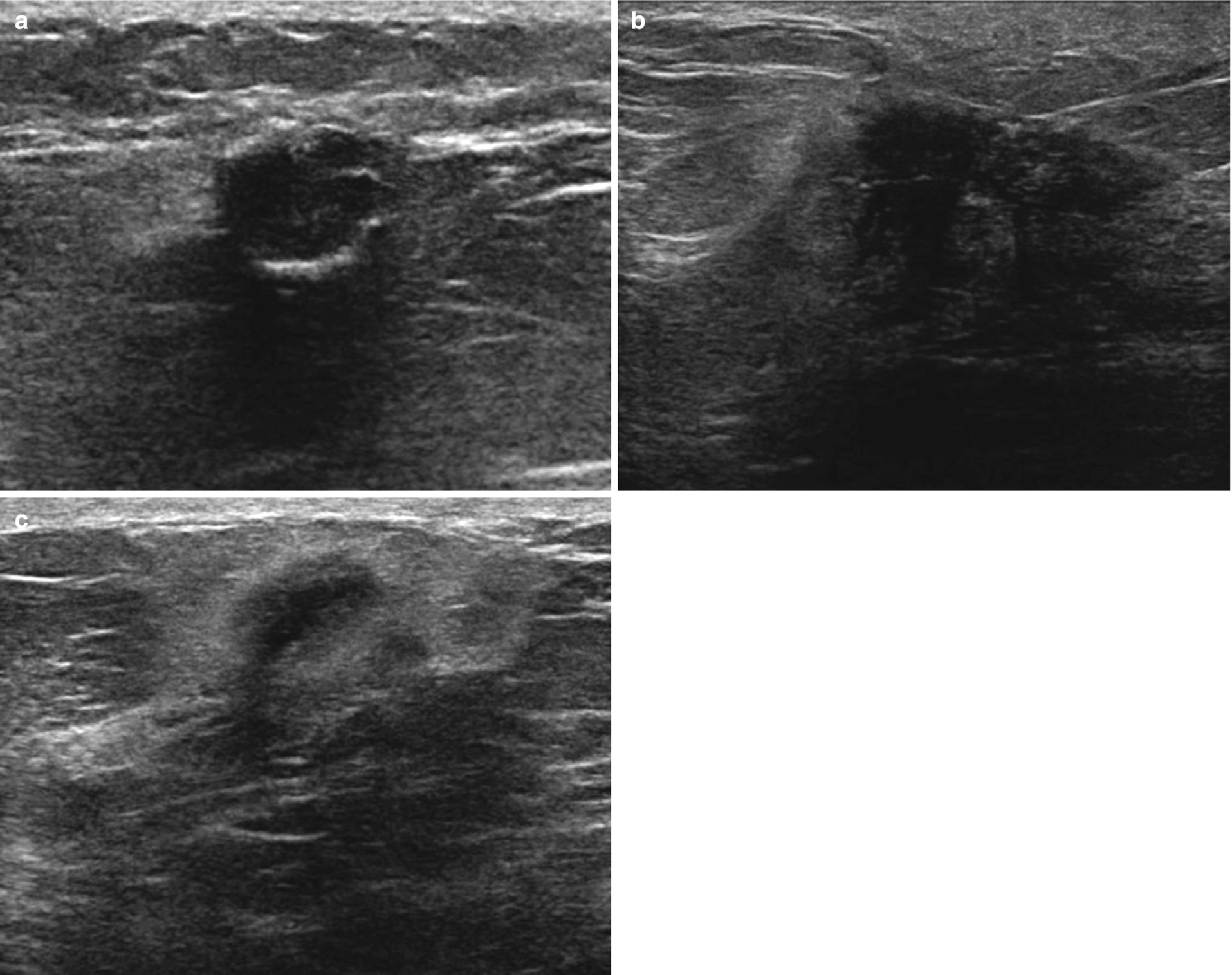
Fat necrosis . (a) Oval circumscribed hypoechoic mass with posterior acoustic shadowing. Corresponding mammography demonstrated a lucent mass consistent with an oil cyst. (b) Irregular hypoechoic mass with angular and indistinct margins and posterior acoustic shadowing. (c) Irregular hypoechoic mass with antiparallel orientation and echogenic halo
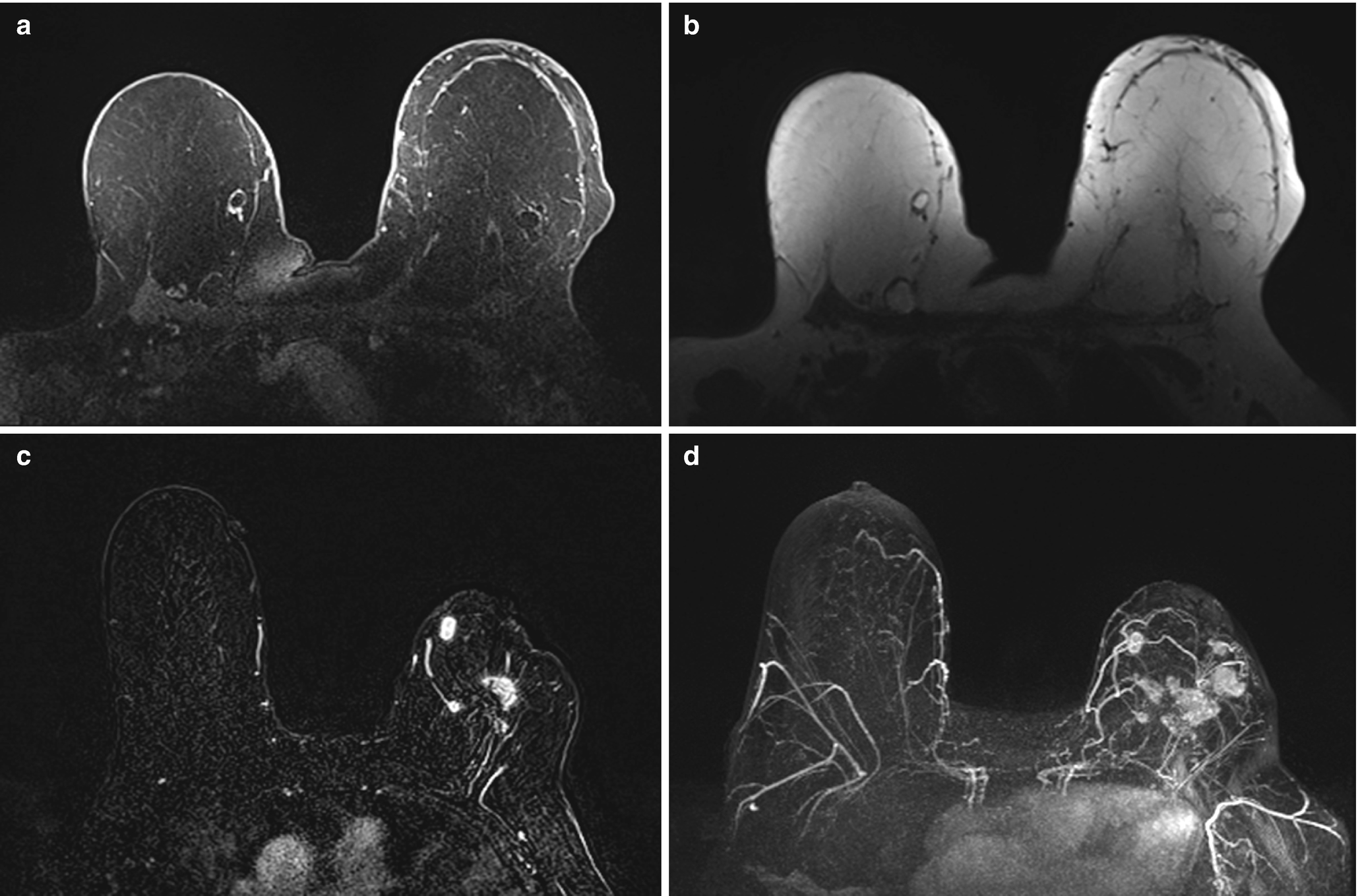
Fat necrosis . (a) Axial T1 fat-suppressed postcontrast image demonstrates two oval rim-enhancing masses in the medial right breast and one in the lateral left breast. (b) Corresponding axial T1 non–fat-suppressed image shows central fat intensity within the masses consistent with fat necrosis. (c) Axial postcontrast T1-weighted MR image demonstrates irregular enhancing masses within the left breast in a patient with history of a left lumpectomy. (d) Corresponding maximal intensity projection (MIP) MR image demonstrates multiple additional irregular enhancing masses within the left breast, biopsy proven fat necrosis
Fine Needle Aspiration
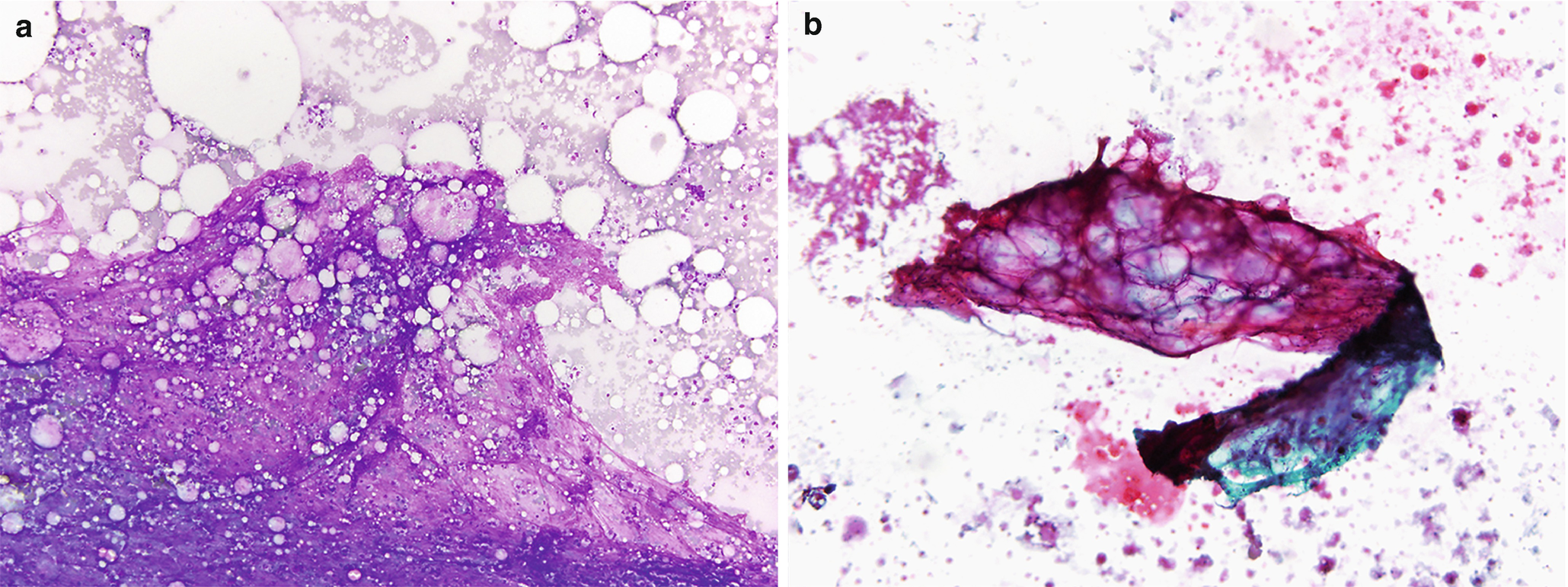
Fat necrosis . (a) Usually hypocellular specimens with numerous macrophages and scattered multinucleated giant cells. Clusters of degenerated adipose tissue, background acellular debris, inflammatory cells, and occasional crystals can also be seen (DiffQuik stain, 100×). (b) Degenerated, necrotic adipose tissue and focal calcifications. These calcifications can sometimes be seen radiologically, and if they arise adjacent to a prior resection site or scar tissue they can raise a concern for malignancy (DiffQuik stain, 100×). (c) The adipose tissue is degenerated, with the majority lacking viable nuclei. Numerous macrophages are scattered between the adipocytes (H&E, 200×)
Core Biopsy
Degenerated adipose tissue with loss of nuclei. Foamy macrophages seen scattered between adipocytes (Fig. 11.6c).
Mastitis
Clinical
Acute: Red, swollen, tender lesion in breast, commonly seen in postpartum period. Secondary to bacteria invading the breast through small defects in the skin of the nipple of a lactating woman.
Chronic: May occur over time if acute infection is not eradicated or can occur de novo and form a mass lesion composed of lymphoplasmacytic inflammation of large and intermediate sized ducts of unknown etiology.
Granulomatous: May arise in a patient with tuberculosis or with a fungal infection or less commonly may be idiopathic in young parous women [1].
Radiology
Mastitis . (a) Right MLO view demonstrating diffuse skin thickening and trabecular thickening as well as an enlarged right axillary lymph node (reactive). (b) US image of the left breast in an area of erythema demonstrates skin thickening and subcutaneous edema without focal fluid collection to suggest abscess. (c) US image demonstrates an irregular hypoechoic mass extending to the skin. Biopsy revealed granulomatous mastitis
Fine Needle Aspiration
Acute: Numerous neutrophils; may see clusters of reactive ductal cells (Fig. 11.8a).
Chronic: Lymphocytes, plasma cells, and amorphous, inspissated debris corresponding to secretions from ectatic ducts.
Granulomatous: Epithelioid histiocytes, multinucleated giant cells, lymphocytes, plasma cells, and eosinophils.
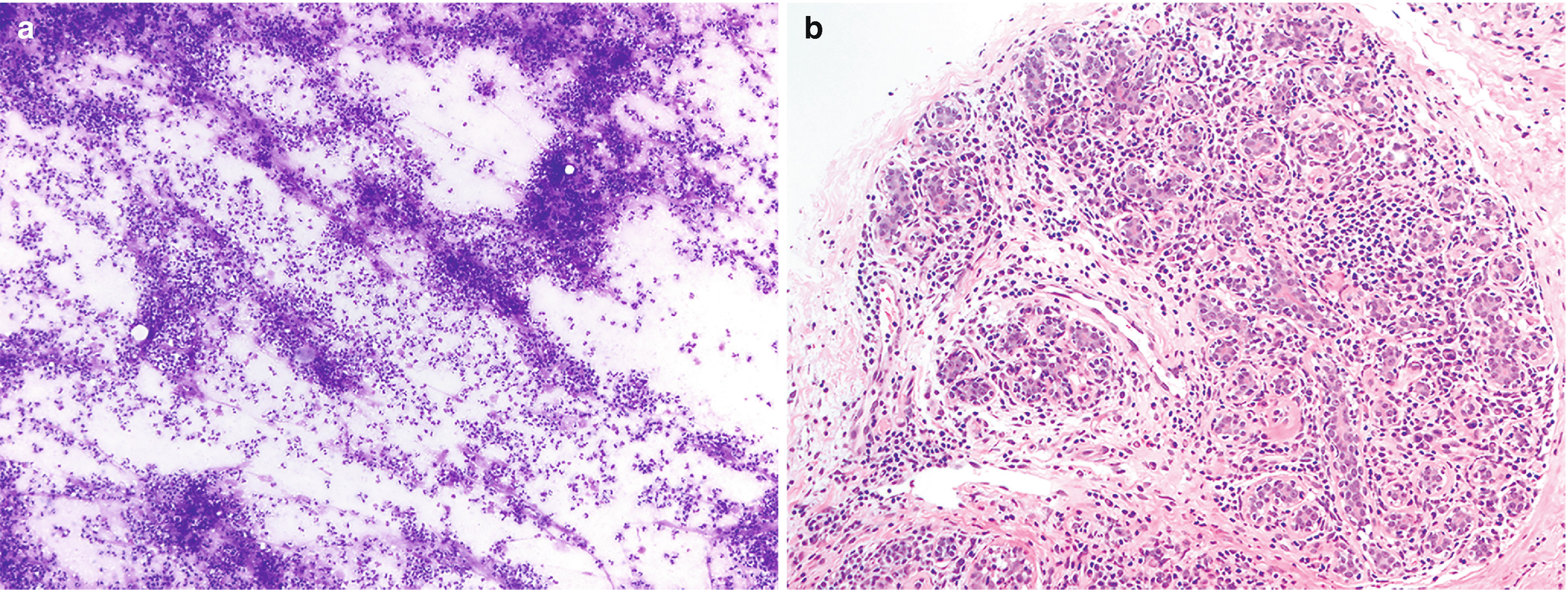
Mastitis . (a) Characterized by sheets of neutrophils. Clusters of ductal epithelium may also be present, but care must be taken not to overinterpret reactive atypia as neoplasia in this particular setting (DiffQuik stain, 100×). (b) Mastitis, chronic lobular type. Breast lobule with abundant infiltrate consisting of lymphocytes and plasma cells (H&E, 200×)
Core Biopsy
Acute: sheets of neutrophils infiltrating breast tissue; bacteria may be seen as well.
Chronic: lymphoplasmacytic inflammation associated with ducts (Fig. 11.8b).
Granulomatous: granulomas, giant cells, and lymphoplasmacytic chronic inflammation.
Subareolar Abscess
Clinical
Painful nodule arising in the subareolar region; can be recurrent.
Aspiration could be performed for microbiology analysis to tailor antibiotic therapy.
Complete excision recommended to prevent chronic infection and development of sinus tracts [3].
Radiology
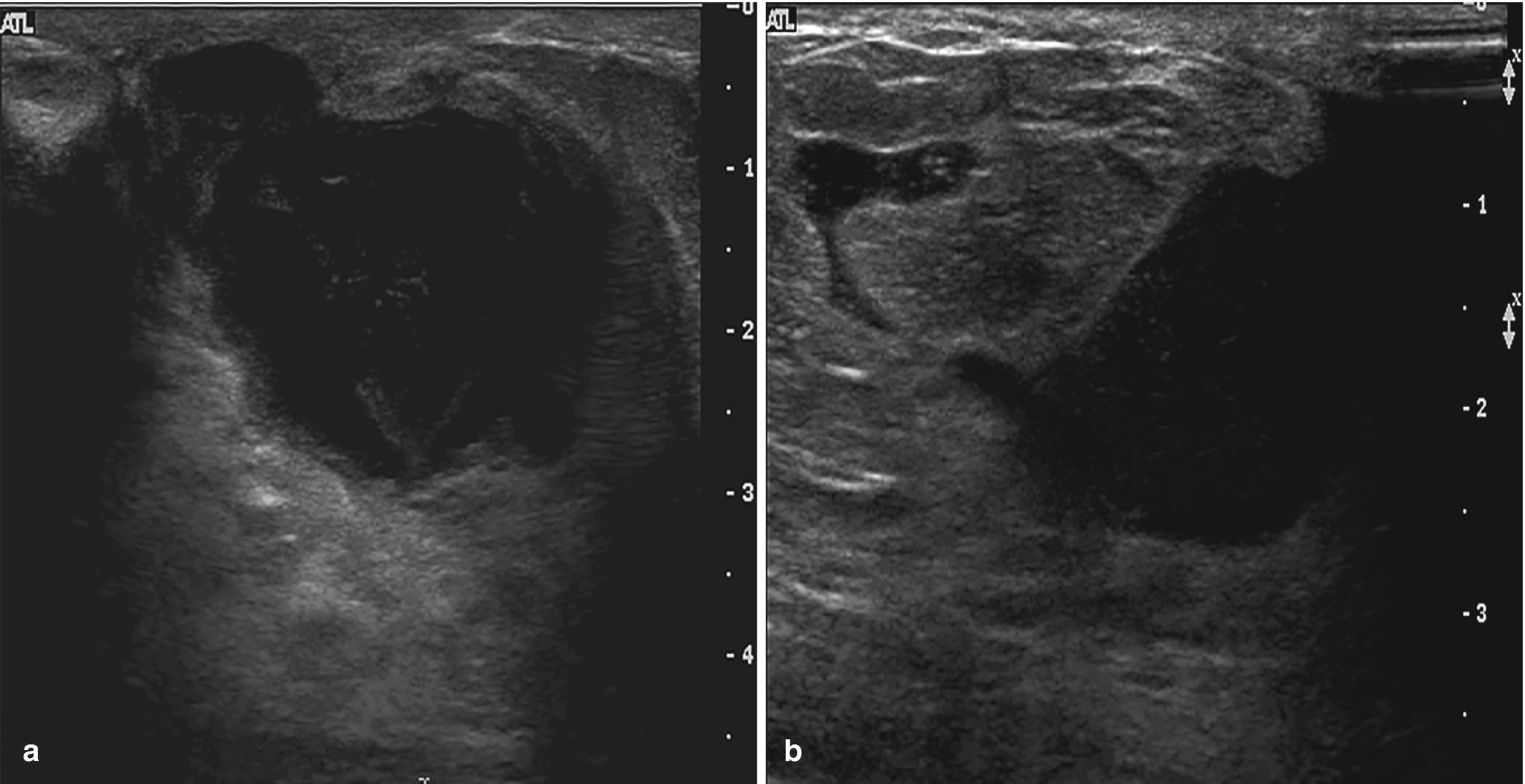
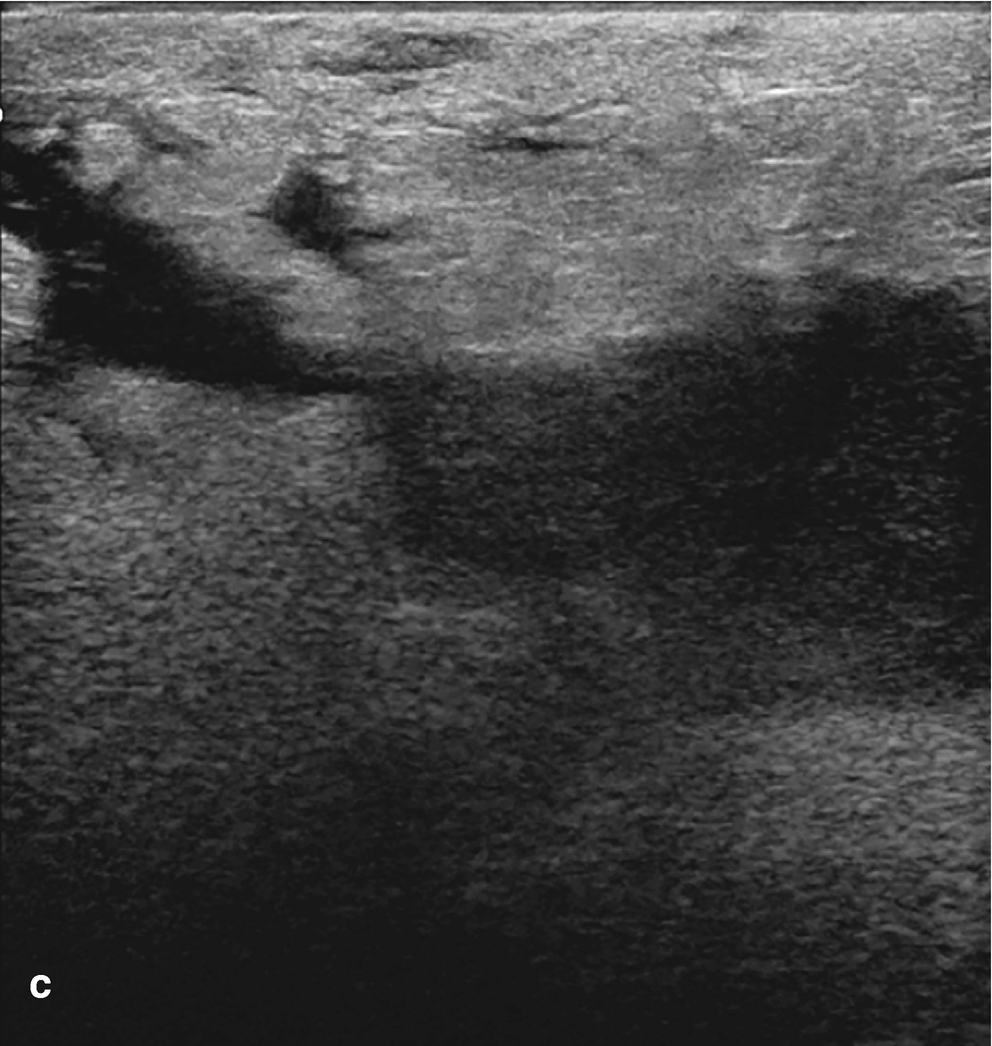
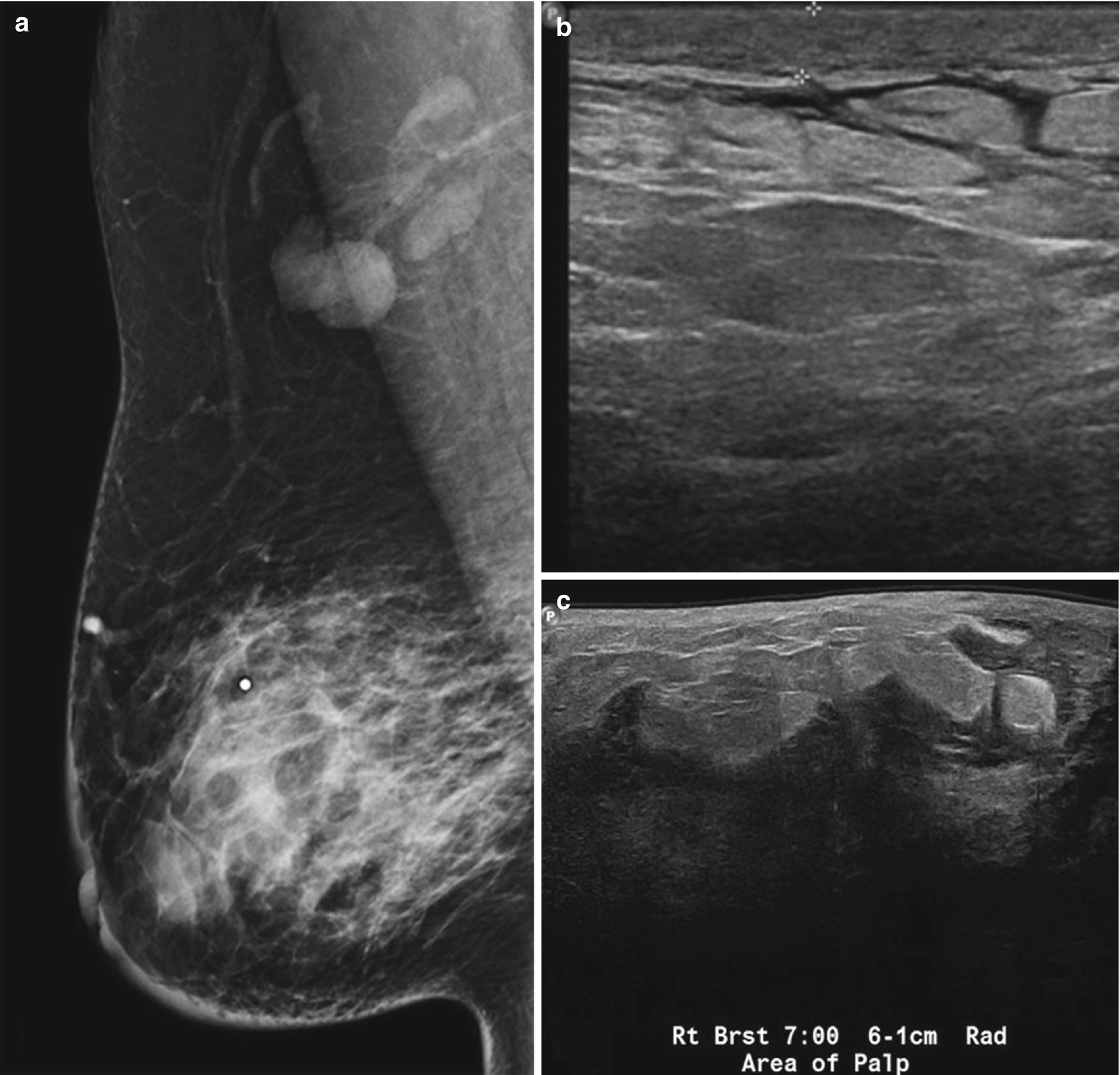
Subareolar abscess . (a) US image of the right breast demonstrates a complex fluid collection at 6:00 in the subareolar breast, corresponding to a palpable abnormality with associated erythema. (b) US image demonstrates a hypoechoic mass in the subareolar breast with infiltration into the adjacent parenchyma. (c) US image demonstrates an irregular hypoechoic mass with extension to the nipple in a patient with symptoms of infection. Overlying skin thickening and edema are also noted
Fine Needle Aspiration
Numerous neutrophils and sheets of anucleated squamous cells (Fig. 11.10).
Histiocytes and giant cells.
Clusters of reactive ductal cells.
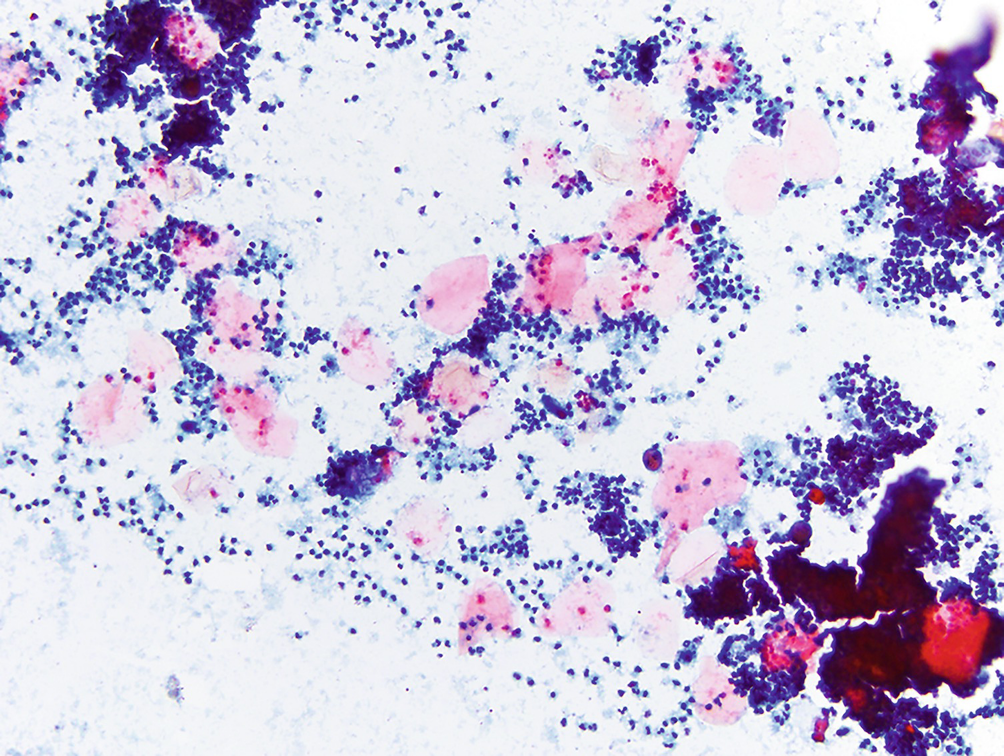
Subareolar abscess . As the name suggests, this lesion is characterized by a usually painful nodule in the subareolar region of the breast. Two main components are present on cytology: numerous neutrophils and sheets of anucleated squamous cells. This condition arises as a consequence of squamous metaplasia and subsequent plugging of the lactiferous ducts. Stasis of normal breast secretions creates a nidus for infection, and over time a red, tender nodule can form (Papanicolaou stain, 200×)
Gynecomastia
Clinical
Most common abnormality of the breast in men.
Enlargement of the breast can be diffuse or nodular and frequently is bilateral.
Can be caused by a number of factors, including low testosterone levels, liver failure, hyperthyroidism, aging, and certain medications, among other less common causes.
Radiology
Mammography
Fan- or flame-shaped density emanating from nipple, usually asymmetric (Fig. 11.11a).

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree