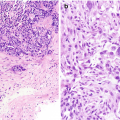
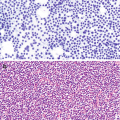
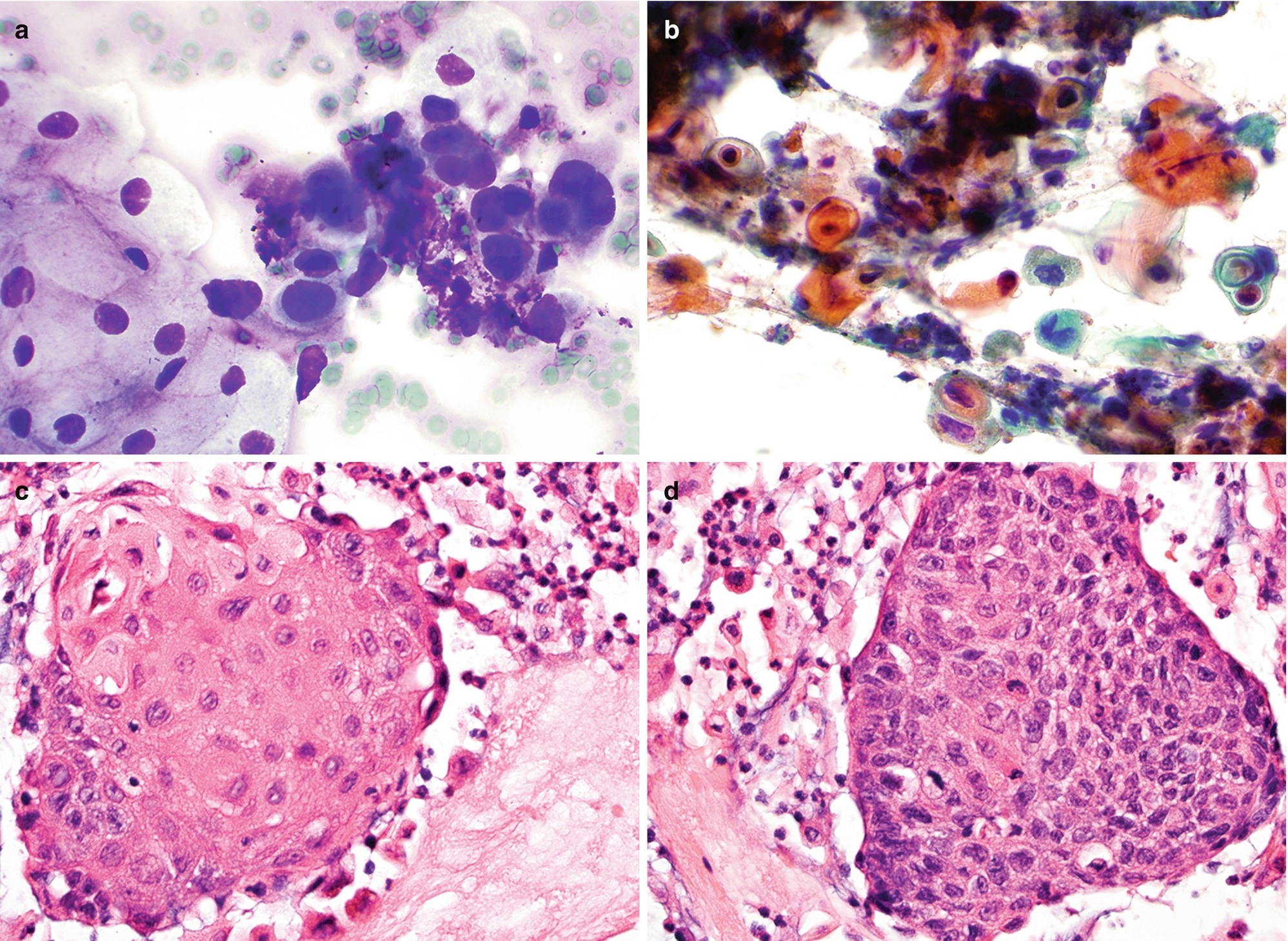
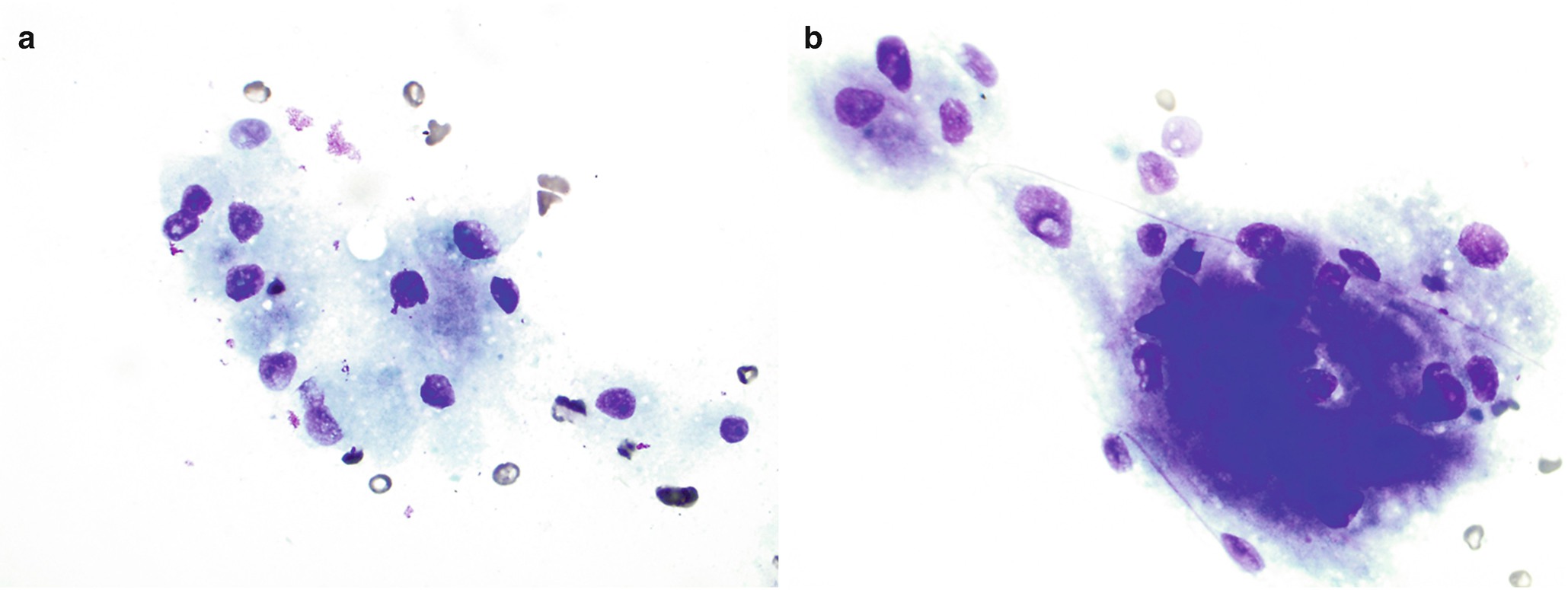
Brushing cytology of gastroesophageal junction, Papanicolaou stain, 600×. (a) Benign glandular mucosa. (b) Barrett esophagus. (c) Low-grade dysplasia. (d) High-grade dysplasia
Histology of Core and Cell Block
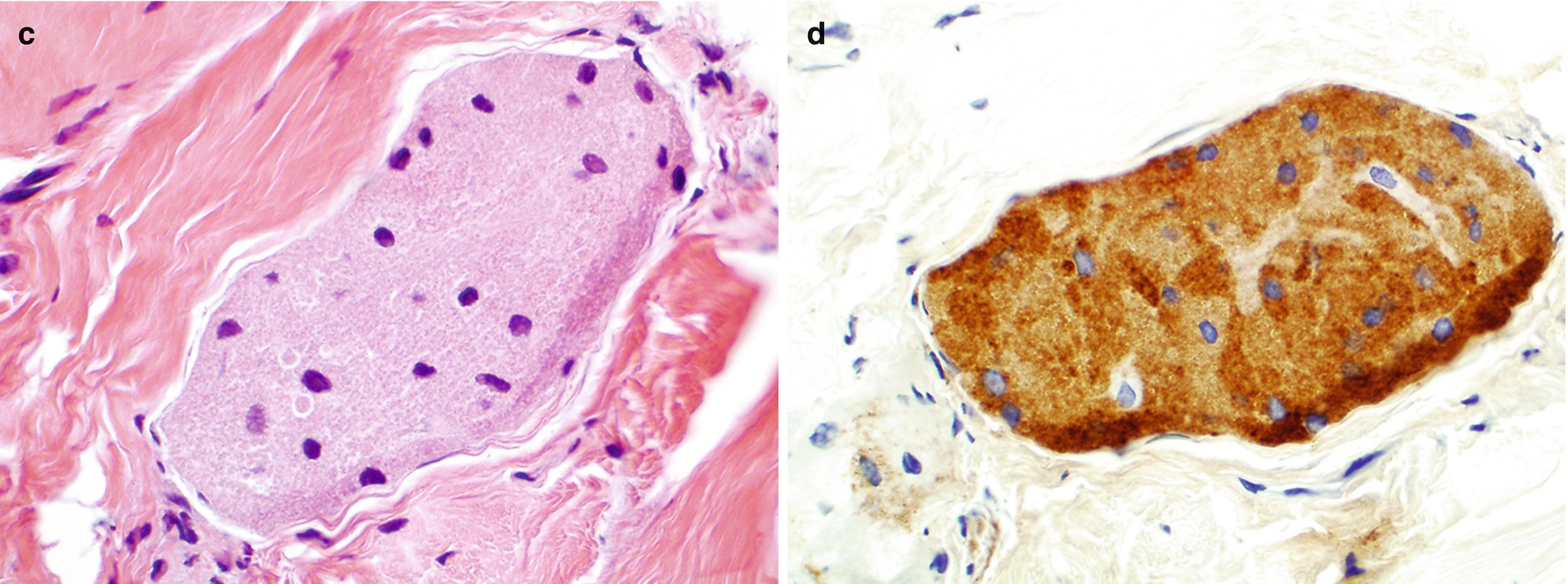
Normal junctional glandular mucosa is composed of cardiac glands (Fig. 7.2a) and/or fundic glands.
Barrett esophagus detected by endoscopic biopsy demonstrates intestinal metaplasia defined by the presence of goblet cells (Fig. 7.2b).
Low-grade dysplasia: Columnar cells with crowded, enlarged, elongated, pseudostratified (confined to the lower half of the glandular epithelium), and hyperchromatic nuclei with mild pleomorphism, increased nuclear-to-cytoplasmic ratio, decreased cytoplasmic mucin, and minimal architectural changes (Fig. 7.2c) [12, 16]. Endoscopic biopsy histomorphology is much more sensitive for diagnosing low-grade dysplasia than brushing cytology (sensitivity 97% versus 20%) [15]. Brushing cytology applied together with biopsy can improve the detection of dysplasia [2, 15].
High-grade dysplasia: Marked architectural aberrations including cribriform glands, marked pleomorphism, nuclear stratification extending to the upper part of the cells and glands, decrease in or loss of mucin secretion, and frequent mitosis (Fig. 7.2d) [12, 16]. Endoscopic biopsy histomorphology and brushing cytology have similar sensitivity and specificity to diagnose high-grade dysplasia (sensitivity 90% versus 90%) and can be used together to increase the sensitivity [15].
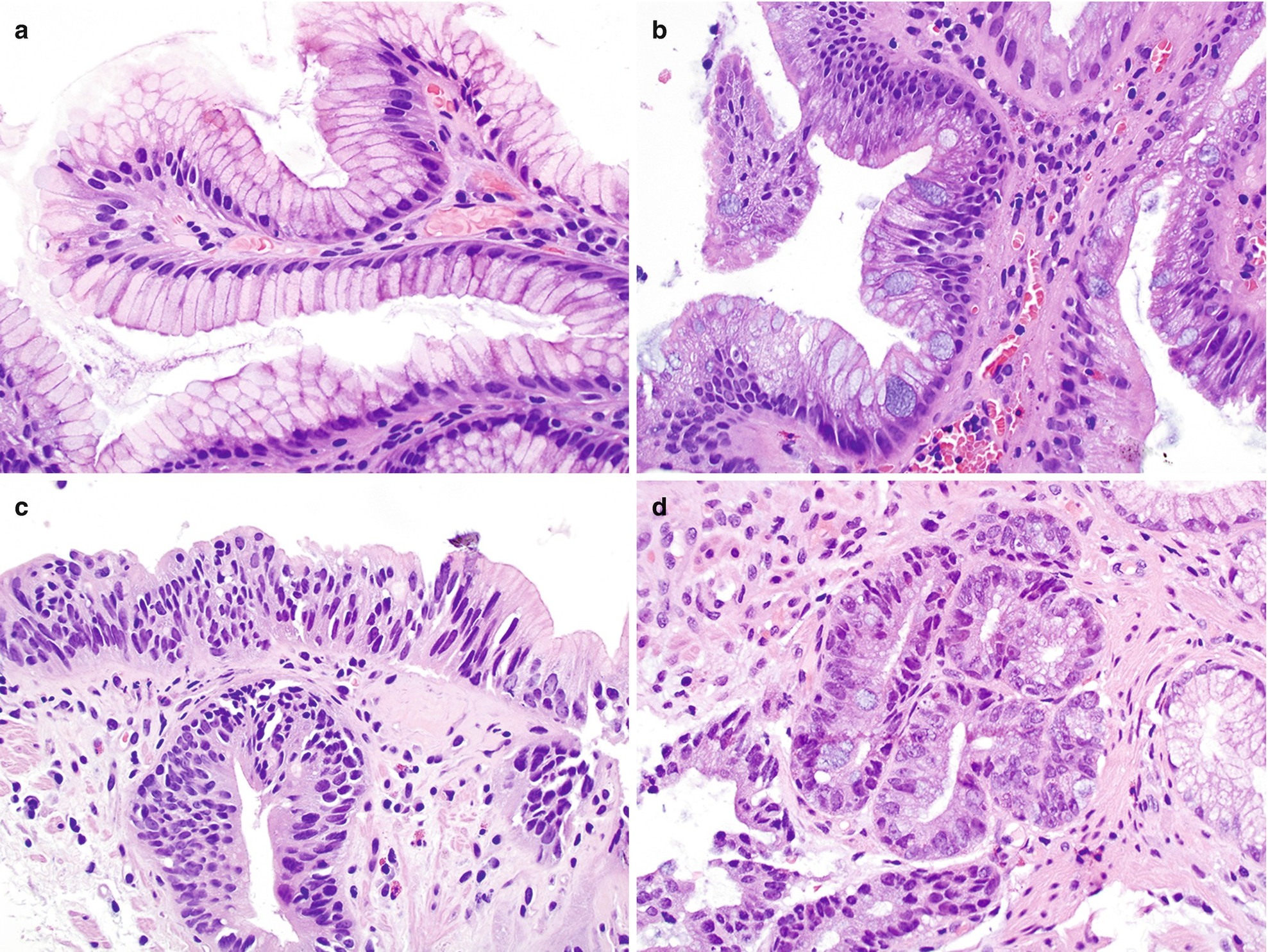
Histology of gastrointestinal junction, hematoxylin-and-eosin (H&E) stain, 400×. (a) Benign glandular mucosa. (b) Barrett esophagus. (c) Low-grade dysplasia. (d) High-grade dysplasia
Carcinoma of the Esophagus and Gastrointestinal Tract
Adenocarcinoma
Clinical
Esophagus
Dramatically increased in incidence in the past two to three decades; approaches or exceeds that of squamous cell esophageal cancer [12].
One to four cases per 100,000 per year in the United States [12].
Male-to-female ratio of 3:1–7:1.
Retrosternal/epigastric pain or cachexia.
Associated with Barrett esophagus in 95% of cases [12]. Other etiologies include tobacco, obesity, alcohol, and Helicobacter pylori [12].
Stomach
The second most common form of esophageal cancer worldwide [18]. Increase in incidence in the past several decades worldwide but markedly decreased in incidence in the United States and England.
Male-to-female ratio of 2:1.
Symptoms include anemia, weight loss, and hypochlorhydria.
Diet, bile reflux, H. pylori infection, excessive cell proliferation, oxidative stress, interference with antioxidant functions, and DNA damage are all causative factors [18].
Two main histologic subtypes:
Intestinal type: Linked to H. pylori, in older patients, and located in antrum.
Diffuse type (signet ring cell carcinoma): Most common in younger patient; located in the body of the stomach.
Radiology for Gastric Adenocarcinoma
Radiography
On a double-contrast barium upper GI study, gastric adenocarcinoma may present as a polypoid, ulcerated, or infiltrative lesion.
When presenting as an ulcerated lesion, the ulcer appears intraluminal as opposed to benign; the latter appears to extend outside the limits of the gastric wall.
Whereas benign ulcers have regular, radiating folds that reach or nearly reach the ulcer margin, malignant ulceration tends to be associated with irregular folds that are separated from the ulcer itself by a rim of malignant tissue (Fig. 7.3a) [19].
CT
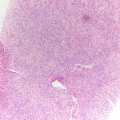
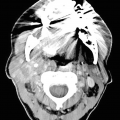
Gastric carcinoma may present as focal or generalized mural thickening or as a polypoid, enhancing lesion (Fig. 7.3b). Advanced carcinomas often demonstrate segmental or diffuse gastric mural thickening with irregular, lobulated borders and ulceration.
Signet-ring cell cancers more often show linitis plastica changes resulting in diffuse thickening of the gastric wall and loss of the normal gastric folds [20].
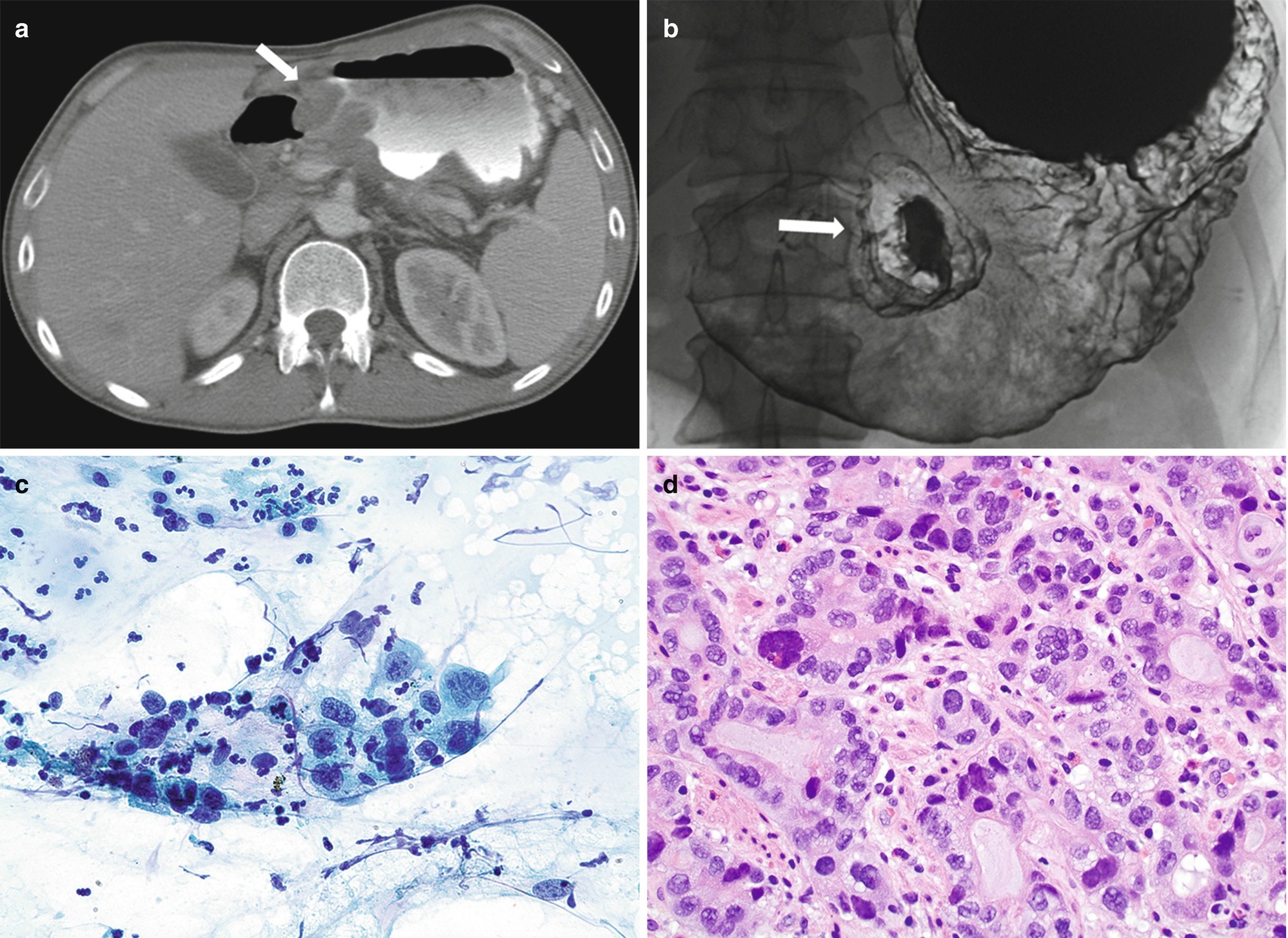
(a, b) Radiology of gastric adenocarcinoma. (a) Spot image from double-contrast upper GI study demonstrating an ulcerated mass projecting into the gastric lumen. Note the thick rind of tissue “heaped up” along the circumference of the ulcer without appreciable gastric rugae (arrow). (b) Contrast-enhanced CT shows marked thickening of the antral gastric wall (arrow). (c, d) Pathology of adenocarcinoma. (c) Brushing cytology, Papanicolaou stain, 400×. (d) Histology, H&E stain, 400×
Gross
Polypoid (type I).
Fungating (type II).
Ulcerated (type III).
Diffuse infiltrative (type IV).
Brushing and FNA Cytology
Brushing cytology has sensitivity similar to that of biopsy for diagnosis of adenocarcinoma (96% versus 91%) [15].
EUS has become an integral component of initial esophageal and gastric cancer staging. The accuracy of EUS for T-staging is approximately 90% [21, 22].
The presence of pleomorphic cuboidal, columnar, or polygonal cells singly or arranged in loosely cohesive and crowded three-dimensional clusters, acini, or papillae is characteristic (Fig. 7.3c) [17]. The nuclei are enlarged and hyperchromatic with abnormal chromatin and one or more prominent nucleoli [17]. The cytoplasm is delicate, granular, or vacuolar and may contain mucin.
Signet-ring cell adenocarcinoma is characterized by the presence of single or loosely cohesive tumor cells with cytoplasmic mucin pushing hyperchromatic, dysplastic nuclei to one side.
Single pleomorphic cells and tumor diathesis are seen.
Histology of Core and Cell Block
Most adenocarcinomas are of the intestinal type arise from metaplastic epithelium. The tumor is composed of irregularly shaped glands that infiltrate deeper layers of the esophagus or stomach (Fig. 7.3d). Tumor cells are cuboidal to columnar with irregular nuclei containing prominent nucleoli and coarse or vesicular chromatin and eosinophilic or clear cytoplasm. Better differentiated tumors are composed of mucin-secreting columnar cells, while poorly differentiated tumors show a solid pattern of growth.
Diffuse type: diffuse infiltration of gastric wall by single tumor cells with associated extensive fibrosis and inflammation. Intracytoplasmic mucin gives cells a characteristic signet-ring cell appearance.
Subtypes:
Adenosquamous carcinoma
Mucinous carcinoma
Hepatoid carcinoma
Parietal gland carcinoma
Lymphoepithelioma-like carcinoma
Sarcomatoid carcinoma
Adenocarcinoma with rhabdoid features
Gastric carcinoma with osteoclast-like giant cells
Immunoreactive to cytokeratin 7, MUC1 (intestinal type), MUC5AC (diffuse type), MUC2 (mucinous type), serotonin and somatostatin (focally positive in 80% of cases), and possibly CK20 (20%) and CDX2.
Differential Diagnosis
High-grade dysplasia
Metastatic carcinoma
Reactive atypia
Neuroendocrine neoplasm
Squamous Cell Carcinoma
Clinical
Esophagus
Overall, the most common malignant tumor of the esophagus worldwide with great geographic diversity, <5/100,000 [23].
Male-to-female ratio of 5:1, two to three times more common in African Americans, with median age of 65 [23].
Risk factors:
Tobacco and alcohol use.
Food and water rich in nitrates/nitrosamines.
Various vitamin deficiencies.
Localized predominantly in the middle or lower third of esophagus.
Presents with dysphagia, weight loss, and retrosternal/epigastric pain.
Anus
Incidence of anal carcinoma is increasing in both males and females.
Male-to-female ratio of 3:1.
Presents with bleeding, pain, change in bowel habits, and pruritus.
Human papillomavirus (HPV), largely HPV 16 genotype, detected in up to 85% of anal cancers.
Most commonly located near the pectinate line; 1% to 3% of all distal large bowel cancers.
The majority are nonkeratinizing squamous cell carcinomas.
Brushing or FNA Cytology , Mainly for Esophageal Squamous Cell Carcinoma
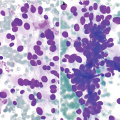
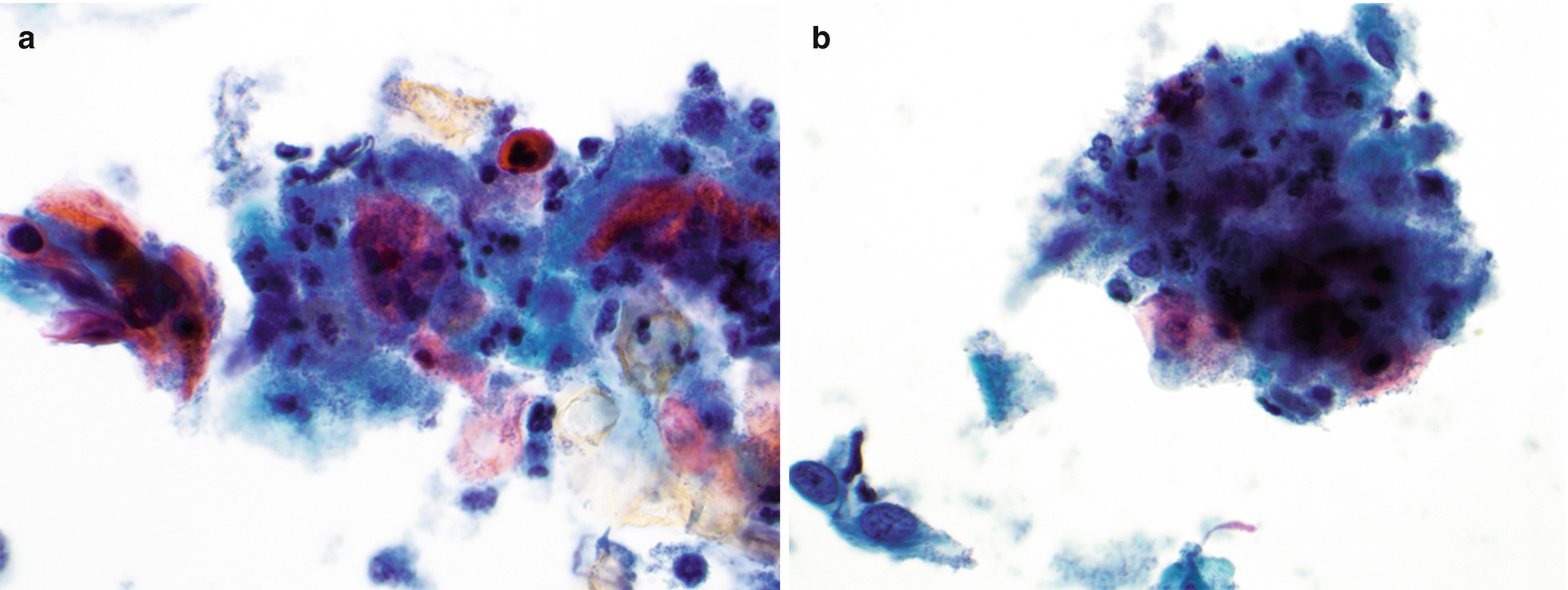
Well-differentiated squamous cell carcinoma (SCC) (keratinizing): Single or three-dimensional clusters of markedly pleomorphic cells (polygonal, bizarre, and spindle in shape) with moderate to abundant dense (keratinized) cytoplasm, slightly enlarged hyperchromatic nuclei, low nuclear to cytoplasmic ratio, and sharp cytoplasmic borders (Fig. 7.4a), in a background of necrosis.
Moderately to poorly differentiated SCC: Loosely cohesive clusters of polygonal cells with enlarged hyperchromatic nuclei containing coarse chromatin and prominent nucleoli and scant to moderate dense cytoplasm (Fig. 7.4b) in a background of necrosis.
Pathology of keratinizing squamous cell carcinoma of the esophagus. (a, b) FNA cytology, Diff-Quik stain (a) and Papanicolaou stain (b), 600×. (c, d) Cell block histology, hematoxylin and eosin stain, 400×
Anal Cytology
Used for screening or follow-up of high-risk patients, analogous to the cervical Pap test.
Dysplastic squamous cells, possibly bizarre in shape with prominent nucleoli, and tumor diathesis (Fig. 7.5).
Cytology of squamous cell carcinoma in anal Pap test, Papanicolaou-stained Thinprep slide, 600 ×
Gross
Most commonly circumferential; often ulcerated lesions with sharply demarcated margins. Polypoid forms are less common.
On cut surface grayish-white tumor invades the esophageal wall and in advanced cases invades surrounding structures.
Histology of Core and Cell Block
Well-differentiated tumors show nests of polygonal epithelial cells with eosinophilic cytoplasm, intercellular bridges with keratinization, and rare basaloid cells (Fig. 7.4c).
Moderately differentiated tumors contain a higher proportion of primitive basaloid cells arranged in irregular nests and trabeculae with focal keratinization (Fig. 7.4d).
Poorly differentiated tumors show no evidence of keratinization and grow in solid sheets or single cells. Large bizarre pleomorphic cells may be present.
Variants of SCC:
Basaloid SCC: cohesive three-dimensional clusters of cells with basaloid appearance (small, round to oval hyperchromatic nuclei and scant cytoplasm), with peripheral palisading and focal mucin secretion or focal squamous differentiation.
Spindle cell squamous cell carcinoma: frankly malignant mesenchymal and spindle-shaped non-keratinizing squamous cells.
Verrucous SCC: A type of well-differentiated SCC.
Lymphoepithelioma-like carcinoma.
Comments
Differential Diagnosis
In situ neoplasia (dysplasia)
Pseudoepitheliomatous hyperplasia
Pseudodiverticulosis
Chemotherapy-related changes
Melanoma (in differential diagnosis of poorly differentiated SCC)
Lymphoma (in differential diagnosis of poorly differentiated SCC)
Immunohistochemistry
Immunoreactive: cytokeratins (AE1:AE3, Cam 5.2), p63, p40, vimentin (some tumors), epidermal growth factor receptor (EGFR), cyclin D1, p16, and p53 expression.
Immunonegative: cytokeratin 7 (only 29% are positive), cytokeratin 20.
HPV tests for anal SCC and some esophageal SCC.
Adenocarcinoma of the Intestinal Tract
FNA cytology is mainly used for staging of tumors. Biopsy under endoscopy is used for diagnosis. The cytomorphology is similar to that seen in the intestinal type of gastric adenocarcinoma, often with a necrotic background.
Neuroendocrine Neoplasm
Low-Grade Neuroendocrine Neoplasm (Carcinoid)
Clinical
Neuroendocrine neoplasm represents 2% of all tumors in the gastrointestinal tract.
The ileum, appendix, and rectum are the most common sites.
Presents with abdominal pain, nausea, diarrhea, weight loss, and in 10% with carcinoid syndrome (flushing, diarrhea, asthma, and tricuspid regurgitation).
Better prognosis than gastrointestinal adenocarcinoma, gastrointestinal stromal tumor (GIST), or lymphoma.
Stomach:
Three main types:
Type I
Associated with autoimmune chronic atrophic gastritis and achlorhydria.
Located in body/ fundus and presents as small, circumscribed nodules.
Type II
Associated with Zollinger-Ellison syndrome, hypertrophic, hypersecretory, and gastropathies.
Male-to-female ratio of 1:1.
Type III
Sporadic.
Male-to-female ratio of 2.8:1.
Located in body fundus, presents as a single nodule, and is larger than 2 cm in 33% of cases.
Small bowel:
The majority occur in adults with a male-to-female ratio of 1.5:1.
Mostly located in the ileum and multiple in 15–35% of cases.
Large bowel:
Almost never associated with carcinoid syndrome.
Appendix:
Most common tumor of appendix.
Peak incidence in 3rd to 4th decades of life.
Gross
Stomach:
Small, sharply outlined lesions with flat mucosa.
Small bowel:
May present as small sessile polyps.
Overlying intact mucosa.
On cross-section, bright yellow in color.
Large bowel:
May present as flat or slightly elevated lesions or as polypoid masses.
May be large and extend deeply through the bowel wall and spread to regional lymph nodes.
On cross-section, bright yellow in color.
Appendix:
Firm, grayish-white well-circumscribed lesions with no capsule.
Cytology of FNA and Touch Preparation
Cellular smears composed of small uniform cells, singly and in loosely cohesive clusters.
Cells have high N/C ratio, delicate or granular cytoplasm, and round to slightly oval nucleus with stippled or fine granular chromatin.
Mitosis is uncommon, and necrosis is not present.
Histology of Core and Cell Block
Stomach
Macroglandular and trabecular or insular (rarely) pattern.
Round to polygonal cells with ill-defined cell outlines and round, monotonous-appearing nucleus with finely stippled chromatin and small nucleoli.
May have plasmacytoid or spindle cell features.
Rare mitosis, absent necrosis, and florid vascularization.
Three main types:
Type I
Small cells with monotonous nuclei, inconspicuous nucleoli, and fairly eosinophilic cytoplasm in microtubular-trabecular arrangement.
Type II
Grossly thickened gastric wall.
Type III
Located in body fundus, present as a single nodule, and larger than 2 cm in 33% of cases.
Appendix:
Classic (insular) type: solid nests of small monotonous cells with occasional rosettes and acini.
Adenocarcinoid: tubular type or goblet cell type.
High Grade Neuroendocrine Carcinoma
High grade neuroendocrine carcinoma includes large cell neuroendocrine carcinoma and small cell carcinoma.
Clinical
Most common in esophagus, especially distal half.
Male to female ratio of 2:1.
Patient presents with weight loss and dysphagia.
Gross
Large polypoid, fungating mass or ulcerating, stenotic lesion
Mesenchymal Neoplasms
Gastrointestinal Stroma Tumor (GIST)
Clinical
Found in middle-aged and elderly individuals.
Most common site is the stomach (more than 2/3 of cases), followed by the small intestine, mostly in the duodenum (1/3 of cases). Only 10% of cases arise in the esophagus, colon, and anorectum.
No sex predilection except for the rare gastric epithelioid tumors arising mostly in females with Carney triad, and in the esophagus where there is a slight male predominance.
Symptoms depend on the size and location of the tumor:
Mostly asymptomatic.
May present with abdominal pain or bleeding, nausea, vomiting, weight loss, and an abdominal mass.
Radiology
US
GISTs often demonstrate heterogeneous echogenicity owing to areas of tumor necrosis. Because of their typically large size at the time of presentation, identification of the organ of origin is often difficult.
CT
GISTs are usually intraluminal or exophytic and often well marginated.
Small tumors demonstrate soft-tissue density on non–contrast images with homogeneous enhancement following contrast administration, although enhancement is typically less than that for normal liver parenchyma.
Large tumors have variable density with heterogeneous enhancement caused by tumor necrosis, which may appear as cystic change, foci of gas, or calcifications (Fig. 7.6a) [25, 26].
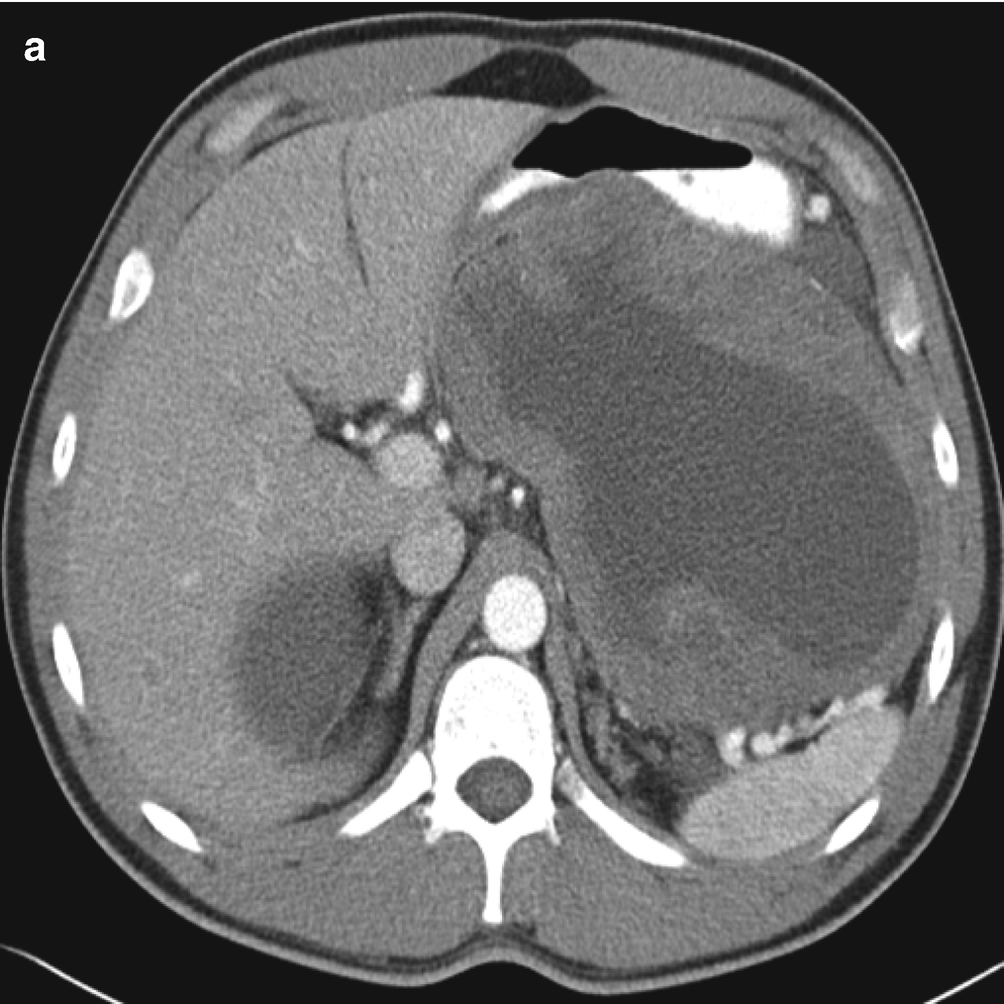
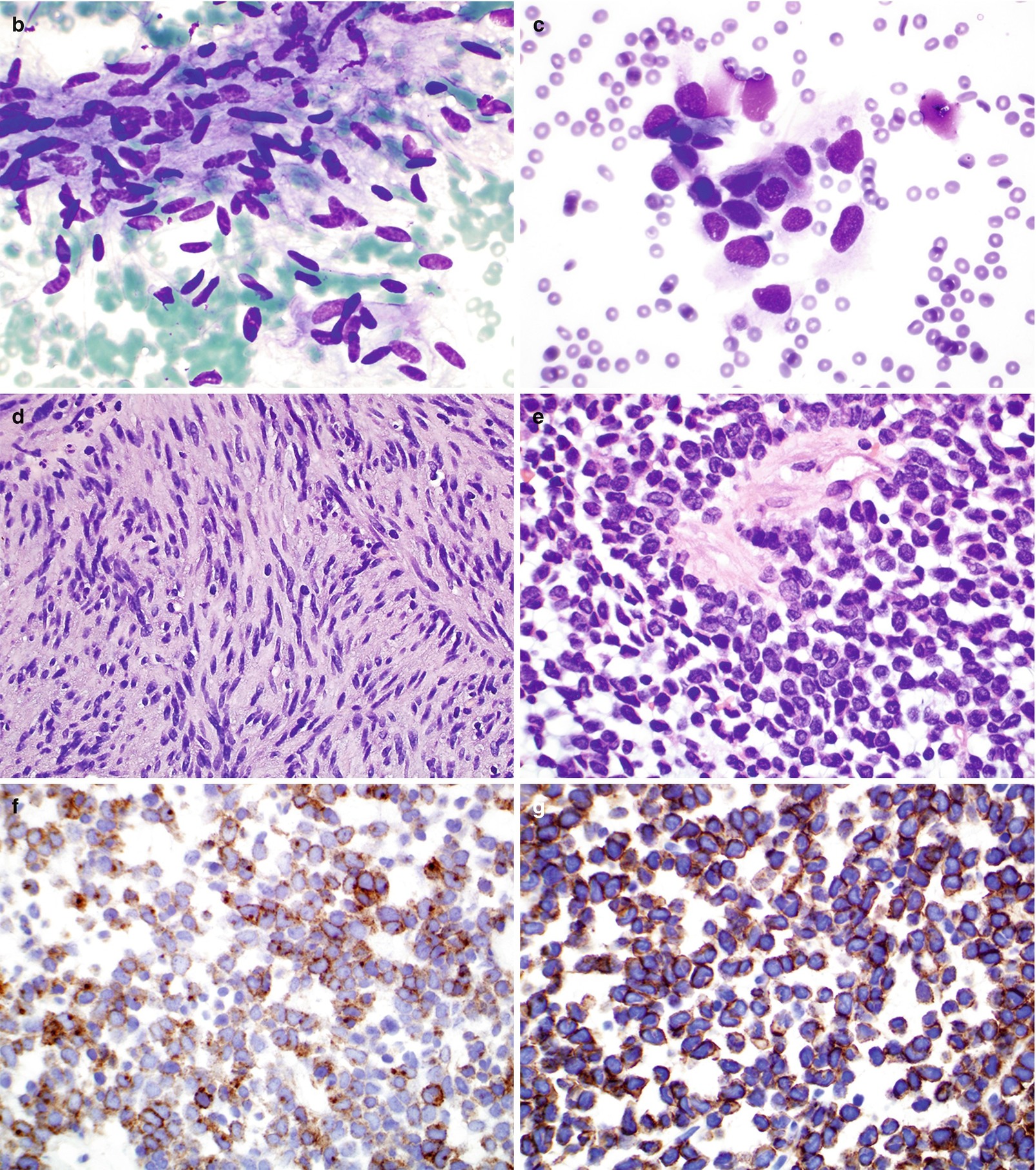
(a) Contrast-enhanced CT of GIST demonstrating a large, heterogeneous mass arising from the posterior stomach. Fluid attenuation centrally indicates tumor necrosis. (b–g) Pathology of GIST. (b, c) FNA cytology, Diff-Quik stain, 600×. (d, e) Core and cell block histology, H&E stain, 400× and 600×, respectively. (f, g) Immunostains for CD117 (f) and Dog1 (g), 600 ×
Gross
Most GISTs are well-circumscribed lesions located in submucosa or the muscularis propria.
The overlying mucosa is either intact or ulcerated.
The cut surface is often granular with areas of hemorrhage, necrosis, and cystic change.
Cytology of FNA and Touch Preparation
Moderate to low cellularity.
Single cells or small aggregate of cells with spindle or ovoid nuclei and delicate scant to moderate cytoplasm with poorly defined borders (Fig. 7.6b, c).
Histology of Core and Cell Block
Spindle cell type (70%) (Fig. 7.6d):
Cells are arranged in fascicles with frequent nuclear palisading and prominent perinuclear vacuoles (fixation artifact) separated by hyalinized stroma.
Densely cellular spindle cells with uniform nuclei with evenly distributed chromatin and abundant eosinophilic to pale cytoplasm.
Epithelioid type (20%) (Fig. 7.6e):
Most common in stomach.
Cells contain rim of condensed cytoplasm around the nucleus with peripheral cytoplasmic clearing.
Composed of sheets of epithelioid cells with round nuclei and small nucleoli.
Mixed type (5%):
Consisting of both spindle and epithelioid tumor cells.
Risk for aggressive behavior is estimated based on the size, location, and mitotic count:
Very low risk: <2 cm and <5 mitosis/50 high-power fields (HPFs).
Low risk: 2–5 cm and <5 mitosis/50 HPFs.
Intermediate risk: <5 cm with 6–10 mitosis/50 HPs, or 5–10 cm with <5 mitosis/50 HPFs.
High risk: >5 cm with >5 mitosis/50 HPFs, >10 cm with any mitotic rate, or any size with >10 mitosis/50 HPFs.
Comments
Differential Diagnosis
Smooth muscle or smooth muscle tumor (leiomyoma, leiomyosarcoma).
Neural tumors (schwannoma)
Fibromatosis.
Leiomyoma
Clinical
Occurs mostly at esophagus and colorectum; very rare in stomach and small bowel.
Most common benign tumor of esophagus, especially lower esophagus.
Males > females (2:1).
Asymptomatic or present with dysphagia.
Gross
Intramural sessile lesion; less commonly present as a polypoid mass.
Up to 10 cm in diameter (usually 2–3 cm).
Solid, gray-white on cross-section; well circumscribed.
Cytology of FNA and Touch Preparation
Fragments of tissue composed of bland spindle-shaped cells of low or moderate cellularity.
The cells contain cigar-shaped nuclei and eosinophilic cytoplasm with indistinct cell borders.
Histology of Core and Cell Block
Mature-appearing smooth muscle cells arranged in fascicles and whorls.
Absent or rare mitosis.
Comments
Differential Diagnosis
Leiomyosarcoma.
GIST.
Immunohistochemistry
Positive: α-smooth muscle actin, desmin, and caldesmon.
Negative: CD117 (C-kit).
Granular Cell Tumor
Clinical
Predominantly in distal esophagus.
Benign behavior; however, rare malignant esophageal granular cell tumors have been reported.
Gross
1–2 cm in diameter, firm yellow nodules or small sessile polyps.
Mostly located in submucosa.
Cytology of FNA and Touch Preparation
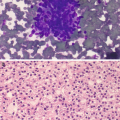
Loosely cohesive large round cells with abundant granular cytoplasm and small, uniform, eccentrically located nuclei (Fig. 7.7a, b).
Pathology of granular cell tumor . (a, b) Touch preparation cytology of core, Diff-Quik stain, 600×. (c) Histology of core, H&E stain, 600×. (d) Immunostain for S100, 600×
Histology of Core and Cell Block
Sheets of oval to large polygonal cells with small central nucleus and abundant granular cytoplasm (Fig. 7.7c).
Immunohistochemistry
Lymphoid Tumors
Clinical
Gastrointestinal tract lymphomas account for 4–18% of all non-Hodgkin lymphomas in Western countries, 25% of cases in the Middle East, and 10% of all gastric malignancies [27]. The stomach is most common site of primary gastrointestinal lymphoma (55–65%), followed by the small bowel and colon.
Predisposing factors are infections (especially with Helicobacter pylori), celiac disease, inflammatory bowel disease, and immune deficiency syndromes [27].
Mostly common are diffuse large B-cell lymphomas, some of which develop through progression from low grade lymphomas of mucosa associated lymphoid tissue (MALT) [27].
Present with abdominal pain, weight loss, obstruction, or as a palpable mass.
Marginal zone B-cell lymphoma:
Women and men equally affected.
88% confined to the stomach, mostly located in distal half.
Occur in individuals older than 50 years.
Diffuse large B-cell lymphoma:
Older adults with slight male predominance.
Some arise in association with marginal zone B-cell lymphoma.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree