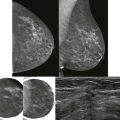
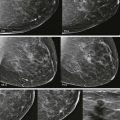
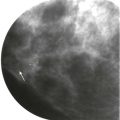
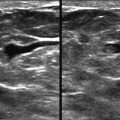
A young woman presented with a palpable lump in her right axilla. She was 3 months pregnant and terrified. Her sister had been diagnosed with stage III breast cancer at age 30. This was her first child. She was thinking the worst. Before starting an ultrasound examination, the patient told us about the lump, she stated that it was enlarging and tender. The ultrasonography (US) showed axillary breast tissue, a normal variant. It was tender and enlarging because of her pregnancy. She left relieved and assured.
Breast anatomy seems pretty straightforward, so why bother to read on? Normal anatomic structures and normal physiologic changes can mimic pathology; recognition of these findings as normal can avoid unnecessary follow-up studies or interventions and reduce patient anxiety. It is also important to be familiar with the normal anatomy as visualized on mammography, US, and magnetic resonance imaging (MRI) so that findings can be correlated among the modalities. Understanding normal breast anatomy and its lymphatic drainage can also help us evaluate the extent of cancers more accurately.
Breast Anatomy
The Fibroglandular Tissue
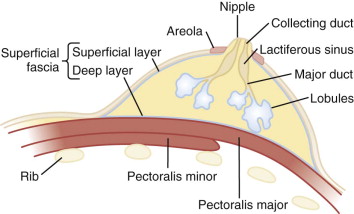
The breast is a mound of fibrous stroma with adipose, ductal, and glandular tissue overlying the anterior chest wall ( Fig. 5-1 ). It often extends to the axillary tail (tail of Spence).
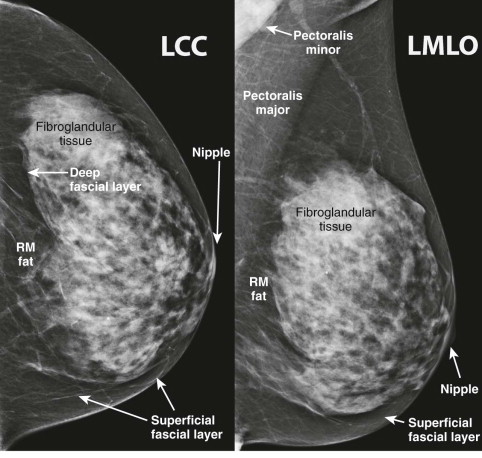
The fibroglandular tissue is surrounded by mostly fatty tissue in the subcutaneous and retromammary (retroglandular) regions ( Fig. 5-2 ). The upper outer quadrant typically contains more fibroglandular tissue than the other quadrants and is where cancers are most likely to develop. The superficial fascia splits into deep and superficial fascial layers that envelop the fibroglandular tissue. The superficial fascial layer lies between the fibroglandular tissue and the subcutaneous fat, while the deep fascial layer is located between the fibroglandular tissue and the retromammary fat. The fascial layers are often difficult to identify on mammography, but may be seen on US ( Fig. 5-3 ). The deep pectoral fascia separates the pectoralis major muscle from the retromammary fat (see Fig. 5-3 ).
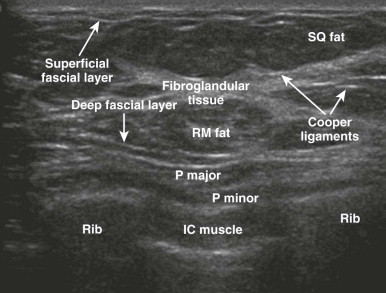
Cooper ligaments are thin sheets of fascia that support the breast. They extend like a mesh through the breast parenchyma, attaching to the dermis and the superficial and deep fascial layers. The Cooper ligaments appear mammographically as fine, white, curvilinear lines throughout the breasts ( Fig. 5-4 ). They are seen on US as thin, curvilinear, echogenic lines (see Fig. 5-4 ). Straightening and tethering of the Cooper ligaments appear as architectural distortion and spiculation, and are often due to invasive carcinoma, radial scar/complex sclerosing lesion, or scarring due to surgery. Retraction of these ligaments by cancers can cause deformity of the border of the fibroglandular tissue, skin dimpling, or nipple retraction. Familiarity with the normal patterns created by Cooper ligaments allows recognition of cases in which these patterns are distorted.

Understanding the typical distribution of fibroglandular tissue can also help detect cancers. In most women, there is little or no dense tissue in the medial and inferior breast or in the retroglandular region ( Fig. 5-5 ). Therefore, developing or focal asymmetry in these areas should be viewed with suspicion.

The border of the fibroglandular tissue has a variable appearance ( Fig. 5-6 ). Abrupt changes in the contour of this border are uncommon in the normal patient. Cancers can produce retraction, protrusion, or spiculation at this border, disrupting the normal tissue contours.

Breast Asymmetry
Asymmetry in breast density or size is usually just a normal variation but may be due to malignancy. There may be considerably more breast tissue in one breast than the other (global asymmetry) ( Fig. 5-7 ). This is a nearly always a normal finding provided there are no breast symptoms such as a palpable lump, breast thickening, or skin erythema, and no associated findings such as architectural distortion. Likewise, asymmetry in the size of the breasts can be quite striking, but is nearly always normal as long as there are no associated clinical or mammographic findings. Beware, however, of the finding known as the “shrinking breast,” which is a sign of invasive lobular carcinoma. This diagnosis should be considered when one breast appears smaller than the other, especially when the parenchyma is dense and the size discrepancy is new or increasing (see Chapter 11 , Expanding the Differential Diagnosis).

Lobules and the Terminal Duct Lobular Unit
The lobules are the milk-producing glands in the breast. They have somewhat of a flower shape ( Fig. 5-8 ). Therefore, lobular calcifications often manifest as a group or cluster of calcifications or multiple clusters (see Fig. 5-8 ). The end branch of the duct and the lobule that it serves is the terminal duct lobular unit (TDLU). Most breast cancers originate in the TDLU.

Ductal Anatomy
A good way to think of ductal anatomy is like a shrub with the base at the nipple. Look at the picture of the tree at the beginning of this chapter. Can you visualize the tree as ductal anatomy? Large branches or major ducts begin to arborize a short distance behind the nipple ( Fig. 5-9 ). These major ducts continue to arborize into smaller and smaller ducts. The branches intertwine, just as branches of a shrub or tree. Ductal systems of the breast are not like segments of an orange or the lobes of the lung that have well-defined anatomic boundaries. A single ductal system can extend over a wide area of the breast (see Fig. 5-9 ). Segmental calcifications involving most of a single ductal system may therefore appear to be regional in distribution.

Ductal carcinoma in situ (DCIS) most often manifests as calcifications within the milk duct. Calcifications that are in a linear or segmental distribution have a higher predictive value for cancer than those with grouped or regional distribution. When a suspicious lesion is identified, the regions between the finding and the nipple and the finding and the chest wall should be scrutinized because they are the most likely locations for additional disease ( Fig. 5-10 ).

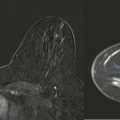
Milk produced in the lobules travels to the nipple via minor ducts that join to form major ducts (see Fig. 5-9 ). The major duct dilates beneath the areola to form the lactiferous sinus. This normal structure is sometimes mistaken for a breast mass on breast self-examination ( Fig. 5-11 ). Milk collects in the lactiferous sinus during lactation. The duct then tapers within the nipple, where it is called the collecting duct (a misnomer, because nothing really collects here).

Nipple and Areola
The nipple has five to ten ductal openings. This is important if you are going to perform a galactogram because you will need to identify which duct is producing the abnormal discharge. If the discharging orifice cannot be identified, galactography cannot be performed.
Nipple in Profile
It is helpful to have the nipple in profile on at least one mammographic projection (CC or mediolateral oblique [MLO]). When not in profile, the nipple can be mistaken for a mass. The subareolar anatomy produces a complex mammographic appearance due to the overlapping lines of the converging milk ducts and surrounding blood vessels. The superimposition of these structures can simulate masses or obscure true lesions.
Retraction Versus Inversion
The nipple may be retracted (pulled back slightly) or inverted (invaginated into the breast) ( Fig. 5-12 ). These variations are normal for many women. In postmenopausal women, the ducts can become enlarged or ectatic, which can result in mild nipple retraction or heme-negative nipple discharge.

If nipple retraction or inversion is of recent onset, it is concerning for breast cancer. In these cases, we routinely perform spot compression views and US of the subareolar region.
Nipple Enhancement
The nipples often enhance on contrast MRI of the breast ( Fig. 5-13 ). This is a normal finding and should not be mistaken for Paget disease or other breast pathology.

The areola has little to do with breast imaging, except that occasionally women with a swollen Montgomery gland may present for diagnostic evaluation. The Montgomery glands are those little bumps on the areola. They can produce discharge, become infected, or produce milk during lactation.
Blood Supply
The primary blood flow to the breast is via the internal mammary artery (60%) ( Fig. 5-14 ). Additional flow comes from the lateral thoracic artery ( Fig. 5-15 ) and perforating vessels from the intercostal arteries. The internal mammary vessels are a good landmark for identifying internal mammary lymphadenopathy on MRI.


Of note, veins in the breast can be quite tortuous, even simulating a mass. Thrombosis of superficial veins in the breast (Mondor disease) is treated conservatively with no need for anticoagulation. Arterial calcifications can simulate early ductal calcifications.
Lymph System
The majority of lymph generated in the breast (97%) drains to the axilla, with the remainder draining to the internal mammary lymph nodes.
Normal lymph nodes can be quite large in long-axis diameter ( Fig. 5-16 ). The cortex should be thin and the hilum should be fatty. On US, the cortex will typically be a few millimeters thick. The hilum of normal lymph nodes is hyperechoic, but may be hypoechoic centrally (see Fig. 5-16 ).