Breast Cancer
Todd M. Blodgett, MD
Alex Ryan, MD
Barry McCook, MD
Key Facts
Imaging Findings
Lesions < 1 cm difficult to detect on whole-body PET/CT or scintigraphy
Primary lesion: Focal increased activity on PET/CT corresponding to suspicious mammographic lesion or lesion on ultrasound
Metastatic disease: Axillary, internal mammary, and distant lymph nodes (LN); bone, liver, and lung most common metastatic locations
PET/CT: Optimal detection of distant metastases in high risk patients; more sensitive for detecting osteolytic bone metastases
Recent evidence suggests can proceed to full axillary lymph node dissection when multiple nodes positive in the axilla
Refinement of locoregional assessment and detection of occult distant metastases in stage II/III disease
Dual time point imaging not universally accepted or used but may be helpful in certain patient populations
Top Differential Diagnoses
Infection/Inflammation
Trauma and Surgery
Lactating Breast
Nonmalignant Tumors
Diagnostic Checklist
PET/CT excellent for staging patients with potentially aggressive breast cancers and for monitoring response to treatment
PET/CT demonstration of osseous metastases usually precludes need for bone scan
TERMINOLOGY
Abbreviations and Synonyms
Ductal carcinoma
Lobular carcinoma
Breast cancer
Breast carcinoma
Inflammatory breast cancer
Paget disease
Definitions
Primary malignancy of breast tissue
IMAGING FINDINGS
General Features
Best diagnostic clue
Primary
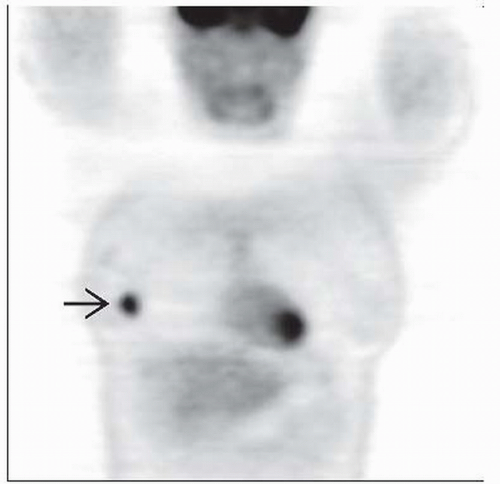
Correspondence between suspicious lesion on mammography/ultrasound (US) and focal uptake on FDG PET
Incidental focal FDG activity on PET or PET/CT should be further evaluated with mammography/US and biopsy
Metastasis
Uptake and morphologic changes in axillary, internal mammary, and distant lymph nodes
Most common metastatic locations are bone, liver, and lung
Location
Primary
Within breast parenchyma, sometimes including contiguous skin or intramammary lymph nodes
Metastasis
Location by relative frequency: Axillary lymph node (LN) > internal mammary LN > bone > liver
Metastases may be seen in any location, often unpredictable
Size
Range from microscopic calcifications to large mass
Lesions may grow to several centimeters
Many smaller lesions not visible on CT or PET
Morphology
Imaging Recommendations
Best imaging tool
Mammography
Still the gold standard for screening
Most cases of breast cancer detected on screening exam are stage I; therefore, PET/CT is not cost effective in this group
For patients with suspected advanced disease or otherwise deemed “high risk”, consider PET/CT for overall staging evaluation
Ultrasound (US)
Preferred modality for determining cystic vs. solid nature of suspicious lesions found on mammography
Image-guided biopsy
US, stereotactic devices, and MR are employed to sample tissue for pathologic evaluation
CT
Dynamic contrast-enhanced CT for detection of intraductal extension of breast cancer
Generally more useful for assessment of spread than for imaging of the primary lesion
3D CT imaging can provide useful information for surgical planning
PET/CT: Lymph nodes
Twice the sensitivity of CT for abnormal nodal findings in internal mammary and mediastinal regions
Improved detection of disease in internal mammary, sub- and interpectoral, supra- and infraclavicular, and Berg level III nodes
Although its sensitivity is lower, the PPV of PET is nearly 100% for detecting malignant nodes
When axillary lymph nodes are positive on PET/CT, may obviate the need for sentinel lymph node scintigraphy
PET/CT: Locoregional disease
May help detect multiple primary tumor sites
Location of primary in patients with breast cancer metastases and indeterminate mammography
Replaces biopsy in patients for whom this is undesirable
Increases confidence of locoregional assessment in stage II/III disease
PET/CT: Distant disease
Whole-body staging is improved, helping to avoid unnecessary surgery
Early detection of bony involvement to help avoid fracture
Best for detection of osteolytic bone mets (bone scan preferred for detection of osteoblastic mets)
PET/CT: Treatment monitoring
Baseline tumor SUV can be established for accurate assessment of therapy response
MR
Modality of choice to evaluate for brain metastases and confirmation of hepatic metastases
Also used in some patients to look at bilateral breast involvement in high risk patients
Used as a problem solving tool in other patients with dense breasts or other processes in which mammography is less sensitive
Protocol advice
10-15 mCi (370-550 MBq) F18-FDG IV
Supine whole-body PET/CT, usually with arms up
Prone imaging
May increase sensitivity when performed following supine study
Improved sensitivity for evaluation of breast, axilla, and mediastinum
Time point of imaging is controversial
Standard is 30-60 minutes after FDG injection
Inflammatory lesions can take up FDG more quickly and intensely than tumor, obscuring evaluation of malignant foci
Increased uptake of tumor over 1-3 hours
Decreasing nonmalignant tissue uptake at these time points
Dual-time point imaging may help avoid inaccuracies imposed by several factors
Serum glucose, insulin, injection-acquisition interval variability, and partial volume effects may all affect image quality and FDG uptake by cells
Use of dual-time point imaging recommended for patients whose breast masses show mild uptake on initial PET images
Dual-time point imaging not routinely used
CT Findings
NECT
Useful for lung & pleural metastases
Can also detect lymphangitic spread
Suboptimal for organ evaluation
CECT
Useful for evaluating mediastinal & organ metastases, particularly in the liver
Lesions appear attenuating compared with fatty background
May show early enhancement on arterial phase on dynamic contrast-enhanced CT
Tumors appear as dense lesions on CT and usually show early contrast enhancement similar to that seen with dynamic MR
CT performance parameters
Sensitivity, specificity, and accuracy in detecting intraductal spread or DCIS: 71.9%, 83.3%, and 76.0%
Sensitivity, specificity, and accuracy for diagnosing muscular invasion: 100%, 99%, and 99%
Sensitivity, specificity, and accuracy in diagnosing skin invasion: 84%, 93%, and 91%
Sensitivity rate for microcalcifications: 59%
3D CT shown to depict and define extent of nearly all tumors in most patients
Nuclear Medicine Findings
PET/CT: General
Sensitivities for PET and PET/CT range from 80-90% for evaluation of primary tumors
Lower sensitivity for smaller primary lesions
60-80% sensitivity for lesions ≥ 2 mm
Prone PET/CT may allow detection of smaller lesions (5-7 mm)
Superior resolution may be afforded by positron emission mammography (PEM), which can detect lesions as small as 2 cm
Sensitivity 90% and specificity 86%
Still investigational
High lesion SUV seen in
Larger invasive tumor
Higher histologic grade, mitotic counts, and nuclear atypia
Absence of hormone receptors
Presence of c-erbB-2 expression
Metastasis to lymph nodes
Infiltrating ductal type (vs. infiltrating lobular type)
Initial Diagnosis
PET/CT is not recommended for initial diagnosis but may be helpful in select patient populations or when standard modalities are ineffective
Consider for occasional use in patients with implants
Dense breast tissue can render mammography nondiagnostic
Cross-sectional morphologic imaging may be equivocal
Lower FDG uptake seen in well differentiated and lobular carcinomas compared to other breast cancers
Normal-range SUV in these malignancies can lead to false negatives
If CT shows a spiculated enhancing mass with low level FDG activity, may represent non-FDG-avid malignancy
Tubular cancer may also have have low FDG uptake
High grade DCIS may be positive on PET if > 1.5-2.0 cm
Tumors with higher tendency to relapse often have SUV above 3.3-4.0
Staging
Overall, whole-body PET/CT limited in detection of < 8 mm lesions
Although not currently recommended for axillary nodal evaluation, positive axillary lymph node on PET/CT has high PPV for malignancy
Characterization of axillary metastases depends on several factors
Size and number of lymph nodes
PET/CT has lower sensitivity of 60-80% for axillary mets
FDG PET can provide resolution only to level of 6-8 mm lesions
Optimal axillary staging depends on sentinal LN biopsy
Evaluation of internal mammary and mediastinal lymph nodes
PET/CT superior for detection and localization (vs. CT and MR)
Accurate staging of these lymph node stations is crucial for prognosis and therapy
PET/CT has 80-95% sensitivity for detecting distant metastases at the time of initial diagnosis
NPV > 70-90%
PPV lower due to confounding factors such as infection, inflammation, etc.
NPV and PPV both benefit from combined modality PET/CT or MR fusion
Detection of hepatic metastases
Combination of low density lesion on CT and increased uptake on FDG PET is highly suggestive of malignancy
MR can clarify cases with positive FDG PET and negative CT
False negatives may be seen with subcentimeter lesions and low density lesions with nonelevated FDG uptake
False positives most often due to infection/inflammation or interposed colon
Occasionally seen incidentally on bone scan
Detection of osseous metastases
Consideration should be given to performing both bone scan and FDG PET/CT at initial staging in high risk patients
Information complementary in breast cancer osseous metastatic assessment
Lytic and trabecular metastases are detected with high sensitivity > 90% on FDG PET
Blastic lesions poorly seen on PET but can be detected on CT
Bone scan is preferred for detection of cortical blastic metastases but has poor sensitivity for lytic or trabecular metastases (75-80% and < 50%)
Effect on management: FDG PET or PET/CT may change patient management up to 51% of the time
PET/CT plays an increasingly important role in radiation therapy planning
Pre-treatment planning or follow-up with PET/CT benefits 40-60% of patients in multiple studies
Restaging
Overall, FDG PET has equal or better accuracy for restaging compared to conventional imaging
Combined PET/CT offers higher sensitivity and specificity than PET alone
False positives due to prior lymphadenectomy
Surgical site may remain positive for 3-12 months
Inflammation may persist surrounding clips or sutures
FDG PET is superior to conventional imaging for diagnosis of metastatic disease (87-90% vs. 50-78%)
In patients with rising serum tumor markers and asymptomatic breast cancer
Response to Therapy
SUV response has proven an accurate indicator of treatment response
Major criterion for good treatment response is approximately 50-60% reduction in SUV following 2 cycles of chemotherapy
> 55% reduction after 1 cycle portends good clinical response
Increase in SUV 7-10 days after antiestrogen therapy may occur due to a metabolic flare
Typically associated with good response
Detection of poor response is equally valuable
Early institution of alternate therapy
Side effects are minimized from inadequate treatments
Other Modality Findings
Positron emission mammography (PEM)
Investigational modality
F-18 used as radiotracer
Improves accuracy for primary lesion detection
F-18 fluoride PET/CT
Superior to traditional bone imaging agents (Tc-99m MDP)
Pending resolution of reimbursement and FDA issues
F-18 estradiol compounds demonstrate whether malignant lesions are estrogen receptor (ER) positive (investigational)
F-18 L-thymidine demonstrates tissue with high DNA turnover (investigational)
DIFFERENTIAL DIAGNOSIS
Infection/Inflammation
Generally lower SUV-to-background ratio than equalsized tumors
Granuloma-producing disease (e.g., sarcoidosis)
Soft tissue infection (e.g., esophagitis, abscess)
Atherosclerosis
Sites of surgical intervention (e.g., resection, ostomy sites)
Intramuscular injection sites
Degenerative bone disease
Non-puerperal mastitis
Trauma and Surgery
Inflammatory uptake related to surgical procedures last 3-6 months
Uptake can be due to hematoma
Scar tissue may demonstrate uptake indefinitely
Traumatic fracture and soft tissue injuries (e.g., lytic bone metastases)
Fibrocystic Disease
Low level FDG uptake may be seen in multiple focal sites
Nonmalignant Tumors
Fibroadenoma, papilloma, and others
Characterized by low level FDG uptake
Hypercellular benign tumors may show increased uptake
Lactating Breast
Glandular tissue may show intense FDG uptake
May see patchy areas of intense FDG activity
History is critical to reduce misinterpretation
Normal Breast
FDG uptake more intense with increasing breast density
Other Malignancy
Second primary neoplasm (e.g., thyroid, lung, colon, etc.)
Primary breast lymphoma
Implants
Inflammatory response to silicone or saline leakage can produce positive PET
Silicone > saline
Calcifications can produce inflammatory uptake or AC artifact (in the case of bulky calcification)
PATHOLOGY
General Features
General path comments: Higher SUV correlates with higher density of viable cancer cells
Genetics
Increased incidence with close family history (e.g., mother, sister)
> 80-85% breast cancer occurs in absence of family history
BRCA-1, BRCA-2
Genetic mutations present in ˜ 0.5% of population
Confer 3-7x risk of developing breast cancer compared to women without these mutations
BRCA-2 may increase breast cancer risk in men
Etiology
Risk factors
Age
Family history
Personal history
Early menarche
Late menopause
Postmenopausal obesity
Radiation exposure (greatest risk with external beam)
Alcohol ingestion
Hormone replacement therapy
Full-term pregnancy at early age reduces risk
Epidemiology
Most common cancer in women
Second to lung as most common cause of cancer death
Lifetime risk in women for breast cancer: 13.2%
With BRCA mutation: Up to 85%
Microscopic Features
Ductal cancers (arising from ductal cells)
In situ: Ducts containing tumor cells with no stromal invasion
Invasive: Tumor penetrates ductal epithelium and invades stroma
Lobular cancers (arising from lobule cells)
In situ: Lobules containing tumor cells with no lobule wall penetration
Invasive: Stromal invasion by tumor cells
Staging, Grading, or Classification Criteria
Tumor (T) staging for primary breast cancer
TX: Primary tumor cannot be assessed
T0: No evidence of primary tumor
Tis: Carcinoma in situ
Tis (DCIS): Intraductal carcinoma in situ
Tis (LCIS): Lobular carcinoma in situ
Tis (Paget): Paget disease of nipple with no tumor;
Tumor-associated Paget disease classified according to primary tumor size
T1: Tumor ≤ 2 cm in greatest dimension
T1mic: Microinvasion ≤ 0.1 cm in greatest dimension
T1a: 0.1 cm < tumor ≤ 0.5 cm in greatest dimension
T1b: 0.5 cm < tumor ≤ 1 cm in greatest dimension
T1c: 1 cm < tumor ≤ 2 cm in greatest dimension
T2: 2 cm < tumor ≤ 5 cm in greatest dimension
T3: Tumor > 5 cm in greatest dimension
T4: Tumor of any size with direct extension to (a) chest wall or (b) skin
T4a: Extension to chest wall
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree