Chapter 8 Breast Cancer Treatment-Related Imaging and the Postoperative Breast
Combined Clinical and Imaging Workup of Breast Abnormalities
For patients with nonpalpable findings on screening mammography, workup always includes diagnostic mammography. For suspicious calcifications alone, the radiologist usually obtains magnification mammograms, often not needing ultrasound. An exception might be extensive pleomorphic microcalcifications, in which ultrasound might be used to search for masses within the area that could be indicative of invasive cancer, prompting biopsy. However, if there is an image-detected mass, area of architectural distortion, or palpable mass, the radiologist usually uses both mammography and ultrasound to evaluate the abnormality, estimate its size, and direct later biopsy. Breast MRI may be valuable in selected cases, as discussed in Chapter 7. Ideally, the radiologist correlates all physical and imaging findings in the report to form a composite picture of all potential abnormalities and their level of suspicion on mammography, ultrasound, and MRI.
Breast Cancer Diagnosis and Treatment
When a combined clinical and imaging workup leads to a breast cancer diagnosis, treatment planning usually involves a consideration of surgery, chemotherapy, and radiation therapy, with the goal to remove all the cancer from the breast, optimize chances for locoregional control, and eradicate occult foci of metastatic disease via systemic treatment (e.g., hormone therapy, chemotherapy), if indicated. The team of breast imagers, surgeons, medical oncologists, pathologists, radiation oncologists, and breast reconstruction surgeons plan the sequence in which surgery, chemotherapy, and radiation occurs. The pathology report is a key component on which treatment is based. The report states tumor histology; size; estrogen, progesterone, and her2neu receptor status; and lymph node involvement. Traditionally, breast tumors are staged using the TNM (tumor, lymph node, metastasis) Classification on Breast Cancer from the American Joint Committee on Cancer (currently in the 7th edition) (Table 8-1). The treatment plan is based on this classification. A clinical decision algorithm is also available from the National Comprehensive Cancer Network regarding the full spectrum of care; Adjuvant! Online is an Internet-based tool that provides guidance regarding prognosis and the potential benefit of different chemotherapy protocols. Additional tests based on tumor gene signatures are emerging (OncoType DX and MammaPrint) and are the subject of two large randomized trials, one in the United States and the other in Europe. Gene expression profiling may play an increasingly important role in the future; preliminary data suggest improvement in separating high- and low-risk patients.
In general, surgeons perform mastectomy when the entire cancer cannot be excised with a good cosmetic result (as just discussed), if the woman has a contraindication to radiotherapy, or if it is the patient’s desire. Usually, patients are offered ipsilateral breast reconstruction with an autologous tissue flap or a tissue expander after mastectomy, unless there is a medical contraindication to reconstruction (e.g., multiple co-morbidities). Because the contralateral breast is often larger than the reconstructed breast, patients may also need reduction mammoplasty on the contralateral side. Characteristic appearances of reduction mammoplasty and breast reconstruction are discussed in Chapter 9.
If the patient has breast-conserving surgery, she usually undergoes postsurgical whole-breast irradiation to achieve control of residual microscopic disease. Relative contraindications to radiation therapy include pregnancy, previous radiation therapy, and collagen vascular disease (Box 8-1). Axillary nodal involvement is not a contraindication. Six randomized trials of lumpectomy and radiation therapy showed that the frequency of local recurrence and overall survival rates are generally comparable to mastectomy. However, IBTRs are reported in 5% of patients at 5 years and in 10% to 15% at 10 years after completion of therapy. Treatment failures (i.e., IBTR) usually undergo salvage mastectomy.
Invasive IBTR usually occurs in the lumpectomy site or quadrant within the first 7 years, but rarely earlier than 18 months after treatment. IBTR after 7 years will more likely occur in any quadrant, not necessarily at the original site, and is usually considered a new cancer. IBTR near the original lumpectomy site is associated more frequently with systemic relapse than IBTR in other quadrants, which more often reflect a new primary tumor. IBTR is considered more likely in women who have invasive ductal cancer with an extensive intraductal component, residual disease in the breast, extensive DCIS, lymphatic or vascular invasion, or multicentricity, and is more common in younger women (Box 8-2).
Evaluation of Axillary Lymph Nodes
ALND is also problematic from the standpoint of side effects. It exposes patients to the risk of major complications such as lymphedema, shoulder dysfunction, and sensory changes in and around the axilla. To address this problem, routine level I/level II ALND (Table 8-2) has evolved to use the SLN biopsy as an initial screen for nodal involvement in patients who are clinically node-negative.
Table 8-2 Location of Lymph Nodes Draining the Breast
| Level | Location |
|---|---|
| I | Infralateral to lateral edge of the pectoralis minor muscle |
| II | Behind the pectoralis minor muscle |
| III | Between the pectoralis minor and subclavius muscles (Halsted ligament) |
SLN biopsy was initially described for patients with penile cancer, but did not attract much attention until it was broadly adopted for use in melanoma patients. SLN biopsy is performed by injecting a tracer material, either a radionuclide, blue dye, or both into the breast either preoperatively or perioperatively and by looking for evidence of the tracer in one or more sentinel nodes (Box 8-3).
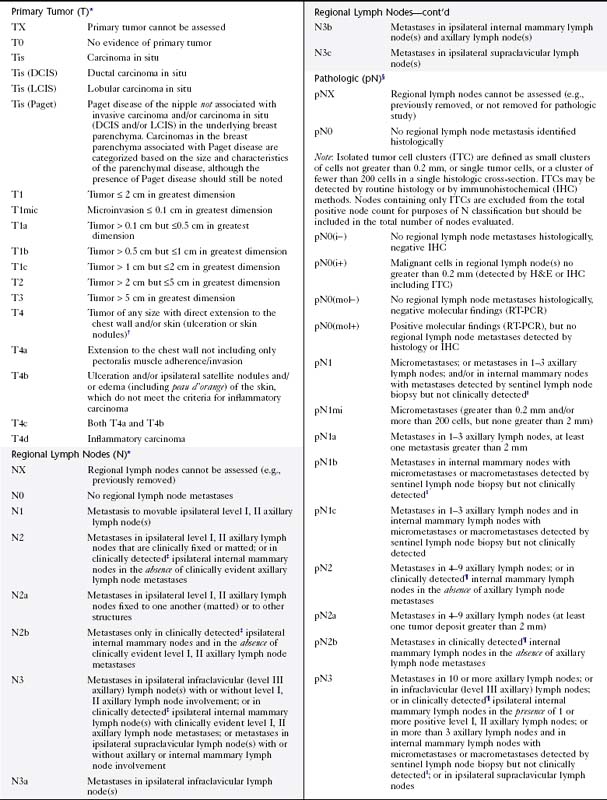
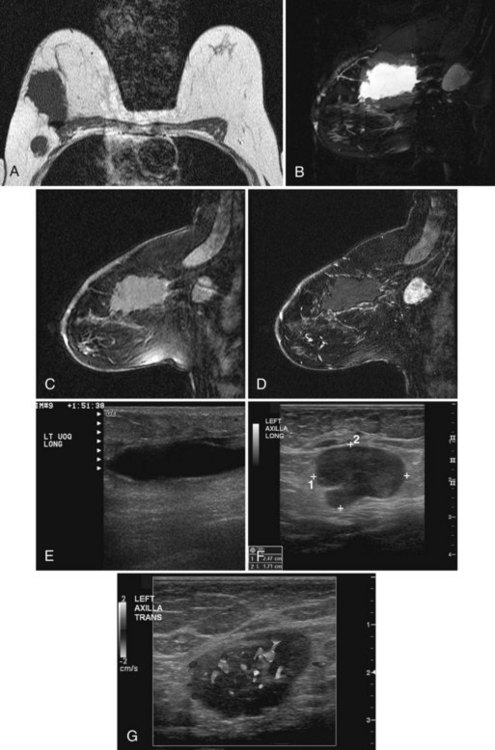
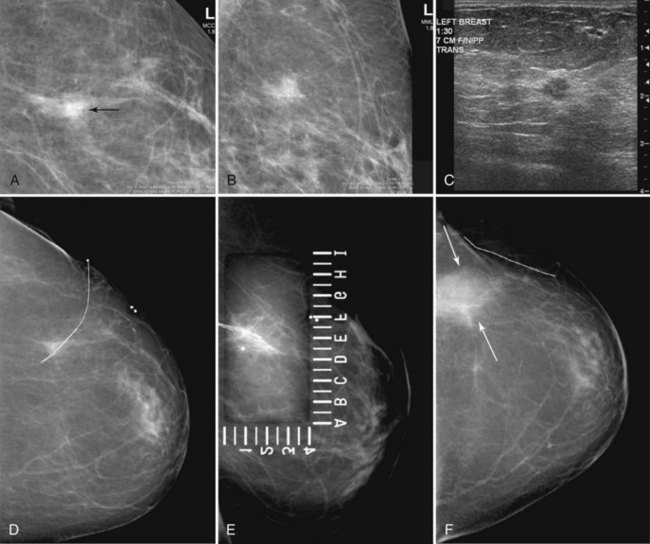
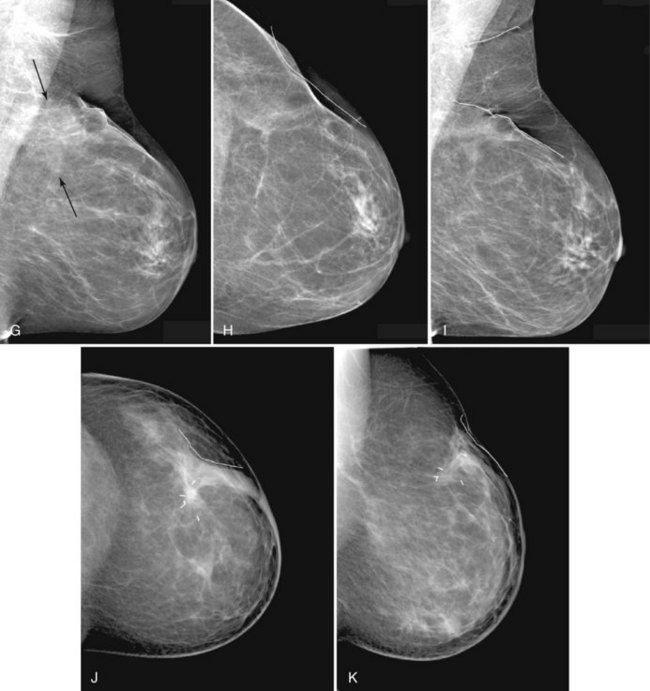
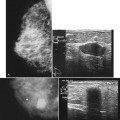
Preoperative lymphoscintigraphy is used in some facilities to assist preoperative localization of sentinel lymph nodes in the axilla or in extra-axillary sites (Fig. 8-1A to C). Most commonly these extra-axillary sites will be in the supraclavicular, infraclavicular, or internal mammary groups. If tracer does not identify an axillary SLN, the surgeon may choose to harvest an SLN from one of these other sites. Some facilities do not remove an internal mammary SLN or other nonaxillary SLN due to the very low frequency of isolated positive biopsies (usually <3%) and the relatively few cases that would result in meaningful changes in prognosis or therapy. Perhaps not surprisingly, institutions that harvest both axillary and internal mammary sentinel lymph nodes have demonstrated a poorer prognosis when lymph nodes at both sites are involved.
One preoperative axillary imaging method that has gained a following is axillary lymph node ultrasound with percutaneous FNA of suspicious nodes (see Fig. 8-1D to G). Although this test is not a routine part of the initial breast imaging evaluation, there is a new appreciation for preoperative evaluation of ipsilateral axillary lymph nodes in the setting of breast cancer. Axillary ultrasound is particularly helpful when the results of clinical examination of the axilla are suspicious for cancer. Several studies have recently been published using ultrasound-guided FNA or core biopsy to document nodal involvement preoperatively, thus allowing the surgeon to bypass SLN biopsy. This can obviate several known issues with intraoperative assessment of sentinel lymph nodes, such as the time needed to harvest one or more nodes, the intraoperative time needed for pathology to evaluate the node and, most important, the potential for false-negative touch preparation or frozen section at the time of surgery, which can lead to reoperation at a later date.
Clinical and Breast Imaging Factors in Determining Appropriate Local Therapy: Lumpectomy or Mastectomy
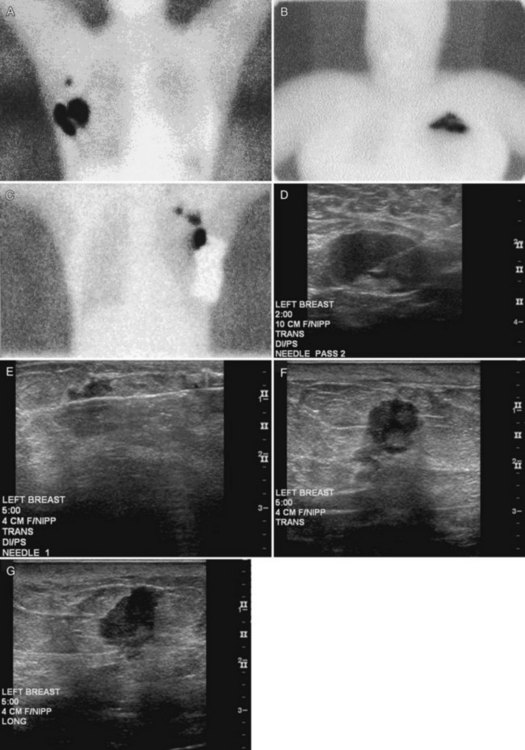
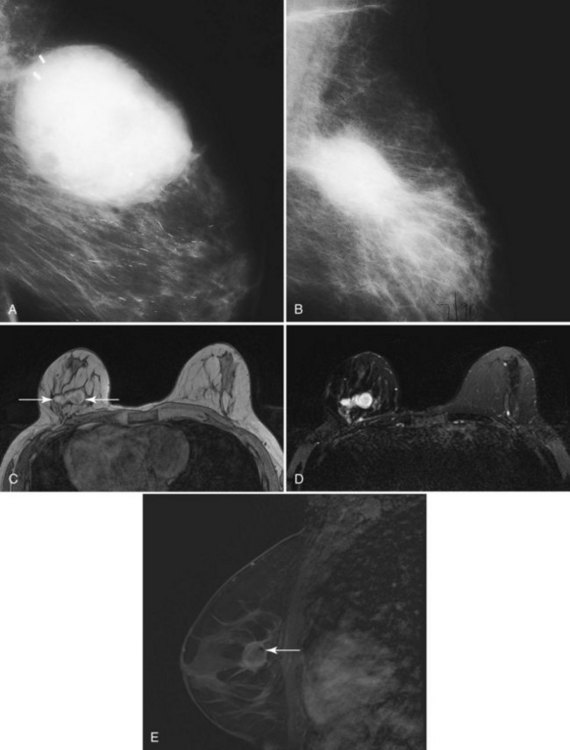
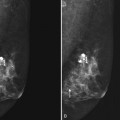
The breast imager plays a critical role in aiding the surgeon to make the right therapeutic choice by showing how much cancer is in the breast. There is virtually no disagreement that patients with a unifocal DCIS or invasive cancer may be treated with breast conservation therapy if the entire tumor can be removed with a good cosmetic result and if there are no relative contraindications to radiation therapy (i.e., pregnancy, collagen vascular disease, poorly defined or multicentric disease) or prior radiotherapy involving the breast (Fig. 8-2).
Preoperative Imaging
Mammography, ultrasound, and MRI for tumor extent are important tools for selecting appropriate breast conservation therapy candidates and planning surgery (Table 8-3). Mammography is the mainstay for determining extent of disease. Mammography identifies diffuse or multicentric disease by finding suspicious breast masses and pleomorphic calcifications. Mammography also can identify benign, extensive, innumerable bilateral calcifications that could hide early tumor recurrence. Such calcifications are a relative contraindication to breast conservation therapy. Furthermore, mammography finds DCIS that is invisible to MRI. Specifically, approximately 25% of DCIS cases are false-negative on MRI and are discovered only by visualizing pleomorphic calcifications on the mammogram.
Table 8-3 Breast Imaging Relating to Breast-Conserving Therapy
| Timing | Reason | Technique(s) |
|---|---|---|
| Preoperative | Ipsilateral tumor extent and contralateral tumor | |
| Establish diagnosis | Percutaneous biopsy | |
| Perioperative | Tumor excision | |
| SLN identification | ||
| Preradiation | Check for residual tumor | |
| Postradiation | Baseline/tumor recurrence | Ipsilateral unilateral mammogram (initial one at 6 mo, then every 6–12 mo) |
| Evaluate ipsilateral and contralateral breast | Bilateral mammogram (12 mo) | |
| Clinical problem |
MRI, magnetic resonance imaging; SLN, sentinel lymph node; US, ultrasound.
Modified from Dershaw DD: The conservatively treated breast. In Bassett LW, Jackson VP, Fu KL, Fu YS, editors: Diagnosis of diseases of the breast. Philadelphia, 1997, WB Saunders, p. 553.
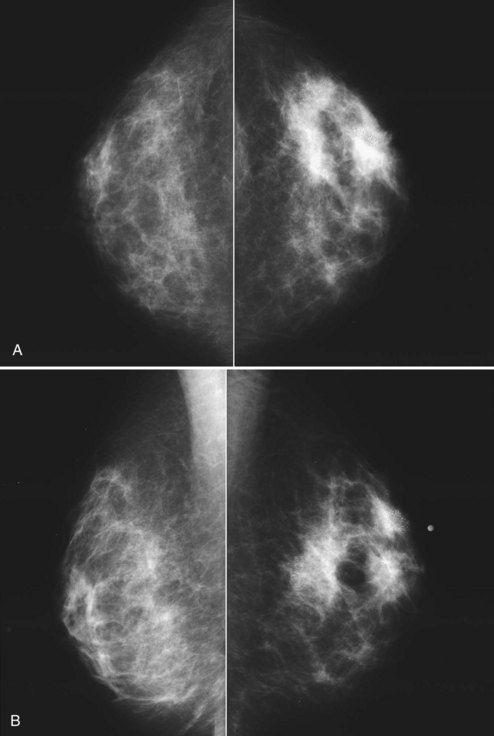
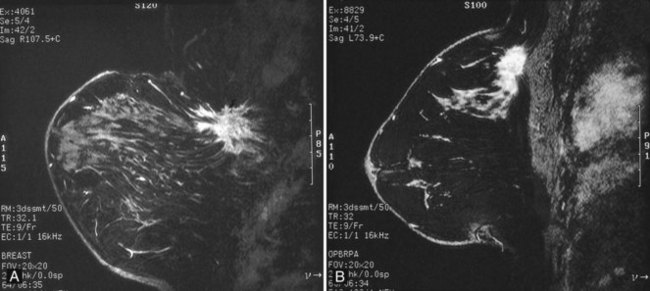
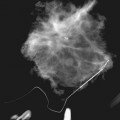
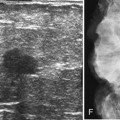
On the other hand, MRI has been especially useful in predicting tumor extent before the first surgical procedure (Fig. 8-3). Some investigators have claimed particular effectiveness of MRI in women with invasive lobular carcinoma or showing tumor invasion into the pectoralis muscle or chest wall (Fig. 8-4). With respect to invasive lobular carcinoma, several studies have suggested that MRI may be more effective in detecting the extent of disease than physical examination, mammography, and ultrasound. However, false-negative studies in these series have led to mixed opinions regarding the routine use of MRI in staging invasive lobular carcinoma.
Chest wall tumor invasion on MRI was shown by obliteration of the fat plane between the tumor and the pectoralis muscle, with muscle enhancement, and was proven in 5 of 5 cases at surgery (Morris et al, 2000). No muscle involvement was seen at surgery when muscle enhancement was absent in 14 of 14 cases.
MRI also helps exclude candidates for APBI when it finds more than one focus of cancer. Bedrosian and colleagues (2003) reported a 95% tumor detection rate with MRI and a change in surgical management in 26% (69/267) of patients requiring wider/separate excision or mastectomy, with pathologic verification in 71% (49/69).
Normal Postoperative Imaging Changes after Breast Biopsy or Lumpectomy
As a rule, mammograms are not often obtained immediately after diagnostic surgical excisional biopsy. However, in the rare cases when a mammogram is obtained within a few days of surgery, mammography shows a round or oval mass in the postoperative site representing a seroma or hematoma, with or without air. This mass represents the biopsy cavity, filled with fluid that should resolve over time (Fig. 8-5A and B). The adjacent breast tissue shows thickening of trabeculae in subcutaneous fat and increased density caused by local edema or hemorrhage. Skin thickening at the incision is usually present. On MRI the biopsy site is filled with blood or seroma. The fluid in the biopsy cavity is high signal intensity on T2-weighted noncontrast fat-suppressed images (see Fig 8-5C to E).
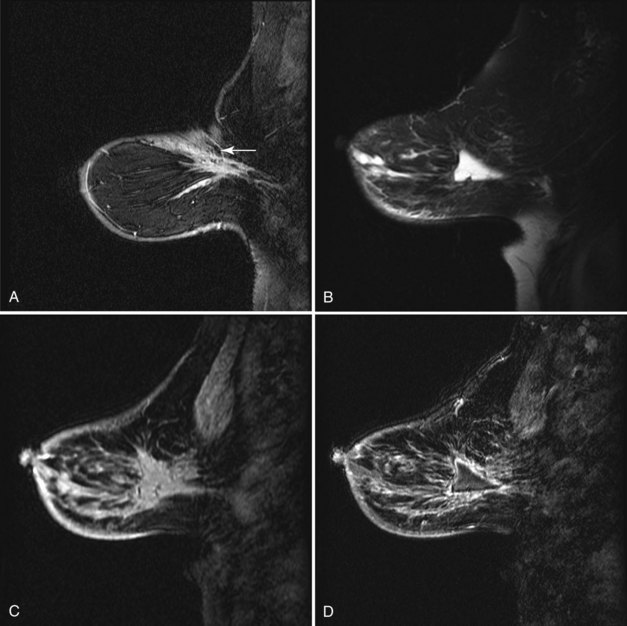
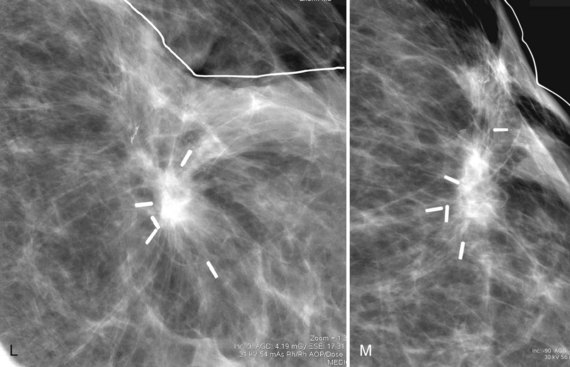
Over the subsequent weeks, the postoperative site resorbs the air and fluid collection; the collection is replaced by fibrosis and scarring, with residual focal skin thickening and breast edema. On MRI the immediate postbiopsy cavity is a fluid-filled structure with surrounding normal healing tissue enhancement for up to 18 months after the biopsy. The biopsy cavity shows high signal intensity, architectural distortion, and a scar that can simulate cancer (Fig. 8-6 and Box 8-4). The biopsy site usually contains fluid from the seroma, which will be bright on T2-weighted images on MRI. Rim enhancement around the biopsy site is normal even if there is no residual tumor and is due to healing. In the ipsilateral axilla, reactive lymph nodes may develop that cannot be distinguished from metastatic disease (Fig. 8-7). MRI after surgery may reveal cancer at the margin edge by showing clumped enhancement or an eccentric residual mass. Although immediate postbiopsy MRI for cancer staging may depict cancer at the biopsy margin, it is more often used to look for cancer elsewhere in the breast away from the biopsy site.
Normal postoperative findings on mammography include architectural distortion, increased density, and parenchymal scarring in at least 50% of patients (Box 8-5). These findings diminish in severity over time (Fig. 8-8A to I). After 3 to 5 years, the findings should be stable on subsequent mammograms. On the mammogram, in 50% to 55% of cases, the biopsy cavity resolves so completely that it leaves no scar or distortion in the underlying breast parenchyma, and only comparison with prebiopsy mammograms indicates that breast tissue is missing. In other cases, the scar appears as a chronic architectural distortion or a spiculated mass more evident on one projection than the other.
Box 8-5
Normal Postoperative Findings for Benign Disease
Increased focal density (edema) near the biopsy site (early)
Oval fluid or fluid/air collection (early)
Complete resolution of biopsy findings (late; 50% to 55% of all cases)
Time when findings resolve: 3 to 5 years after biopsy
Postoperative findings seen after 3 to 5 years (45% to 50% of all cases)
Data from Brenner RJ, Pfaff JM: Mammographic changes after excisional breast biopsy for benign disease, AJR Am J Roentgenol 167:1047–1052, 1996; and Sickles EA, Herzog KA: Mammography of the postsurgical breast, AJR Am J Roentgenol 136:585–588, 1981.