Breast magnetic resonance (MR) is highly sensitive in the detection of invasive breast malignancies. As technology improves, as interpretations and reporting by radiologists become standardized through the development of guidelines by expert consortiums, and as scientific investigation continues, the indications and uses of breast MR as an adjunct to mammography continue to evolve. This article discusses the current clinical indications for breast MR including screening for breast cancer, diagnostic indications for breast MR, and MR guidance for interventional procedures.
Breast cancer is the most common cancer among women. In 2009, approximately 192,370 new cases of invasive breast cancer, and an additional 62,280 in situ breast cancers, will be diagnosed. An estimated 40,170 women are expected to die from breast cancer in 2009. Because of early detection of breast cancers from widespread screening mammography and improvements in treatment, the mortality from breast cancer has decreased almost 30% since 1990.
Mammography is the mainstay of breast imaging. Other imaging technologies such as ultrasound and magnetic resonance (MR) have been found to be useful adjuncts to mammography. Breast MR is highly sensitive in the detection of invasive malignancies, with reported rates of 89% to 100%, although it is somewhat limited with variable specificity, reported at 37% to 97%. As technology improves, as interpretations and reporting by radiologists become standardized through the development of guidelines by expert consortiums, and as scientific investigation continues, the indications and uses of breast MR as an adjunct to mammography continue to evolve.
The specificity of breast MR has gradually improved, likely because of improved technology and increased reader experience. To standardize interpretation among radiologists and to facilitate outcome monitoring, the American College of Radiology (ACR) has developed guidelines for interpretation: the ACR BI-RADS (Breast Imaging Reporting and Data System) MR Imaging Lexicon, the first edition of the MR Imaging Lexicon, in 2003. The BI-RADS MR Imaging Lexicon provides common terminology for describing findings and allows radiologists to communicate findings to referring physicians and with each other in a standardized fashion. It also facilitates comparison of findings across scientific investigations.
One of the current challenges that breast MR faces is that technical parameters are not standardized, varying with equipment and across practice sites, including magnet strength, pulse sequences, spatial resolution, and timing of postcontrast sequences. To address this issue, the ACR has also developed practice guidelines and technical standards for breast MR, the most recent update in 2008. The ACR is also in the process of developing a breast MR accreditation program that will be part of the breast imaging accreditation program and will include technical requirements for optimal breast imaging and the requirement for an MR biopsy program. The current guidelines by the ACR are the foundation for this article on the current clinical indications for breast MR.
The ACR guidelines are designed to assist practitioners in providing appropriate radiologic care for patients. They are not inflexible rules, and the ultimate judgment in determining the appropriateness of specific imaging or a specific procedure must take all circumstances into consideration. MR findings should be correlated with clinical history, physical examination, and results of mammography and other prior breast imaging.
The current guidelines published by the ACR recommend breast MR in the following circumstances :
- ■
Screening for the high-risk patient
- ■
Screening the contralateral breast in a patient with a recently diagnosed breast malignancy
- ■
Screening in patients with breast augmentation, for example in patients with postoperative reconstruction and previous free injections.
The diagnostic indications for breast MR are as follows:
- ■
For extent of disease in patients with invasive carcinoma or ductal carcinoma in situ (DCIS)
- ■
In assessing invasion deep to the fascia
- ■
Postlumpectomy with positive margins
- ■
Following patients with neoadjuvant chemotherapy.
In the additional evaluation of clinical or imaging findings, evaluation for recurrence of breast cancer in metastatic cancer of unknown primary or axillary adenopathy, in which a breast origin is suspected, and in lesion characterization, breast MR may be indicated when other imaging modalities, such as mammography and ultrasound, are inconclusive for the presence of breast cancer and biopsy cannot be performed. Breast MR may also be helpful for the additional evaluation of clinical or imaging findings in postoperative tissue reconstruction. Breast MR is also used for guidance for interventional procedures, such as MR core biopsy and MR-guided wire localization ( Table 1 ).
| Breast cancer screening | Screening for the high-risk patient |
| Contralateral breast | |
| Breast augmentation | |
| Diagnostic indications | Extent of disease in patient with known breast cancer |
| Following patients with neoadjuvant chemotherapy | |
| Breast cancer recurrence | |
| Metastatic cancer of unknown primary or axillary adenopathy | |
| Lesion characterization when mammography and ultrasound are inconclusive | |
| Postoperative tissue reconstruction | |
| Procedure guidance | MR core biopsy |
| MR-guided wire localization |
Screening for the high-risk patient
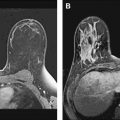
In 2007, the American Cancer Society published guidelines for screening for breast cancer with MR as an adjunct to mammography. The guidelines were based on several major clinical trials, the first of which was published by Kuhl and colleagues in 2000, in which 192 asymptomatic women with proven or suspected BRCA mutations were enrolled in a prospective trial comparing MR with conventional imaging. Nine breast cancers were identified, 4 by mammography and ultrasound (44% sensitivity) and all 9 by MR (100% sensitivity).
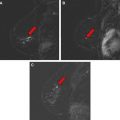
Since then, there have been several studies worldwide showing benefit to screening subsets of women at high risk for breast cancer with MR. In a review of 8 major clinical trials for MR screening of known or suspected BRCA 1 and BRCA 2 carriers in 4271 patients, 144 breast cancers were found with a 3% cancer yield. Overall, there was a high sensitivity of MR for screening in high-risk populations, with a sensitivity of 71% to 100%, compared with 16% to 40% sensitivity with mammography. The specificity was variable. The call-back rate ranged from 8% to 17% (average 10%), with a benign biopsy rate of 3% to 15% (average 5%). Figs. 1 and 2 show malignancies detected by screening MR in high-risk patients.


The patients at highest risk for breast cancer are those with a mutation of a breast cancer susceptibility gene. They constitute only 5% to 10% of women with breast cancers, but a familial genetic mutation confers a cumulative lifetime risk of breast cancer between 50% and 85% (19% risk by age 40 years, 50% risk by age 50 years, and 85% risk by age 70 years). The most common of these are the BRCA 1 or 2 mutations, which account for approximately 40% to 50% of familial breast cancer; the others are caused by other familial genes that cannot be tested for at the present time.
The American Cancer Society in 2007 recommended annual MR screening for breast cancer as an adjunct to mammography, based on evidence, for patients with a known BRCA mutation, patients who are a first-degree relative of a BRCA carrier, but untested, or patients with a 20% or greater lifetime risk, as defined by BRCAPRO or other models that are largely dependent on family history. They recommended annual MR screening, based on expert consensus opinion, for patients with radiation to chest between the ages of 10 and 30 years, patients with Li-Fraumeni, Cowden, and Bannayan-Riley-Ruvalcaba syndromes, and first-degree relatives. They reported that there is insufficient evidence to recommend for or against MR screening in patients with a 15% to 20% lifetime risk. They also concluded that there is insufficient evidence for or against MR screening in patients with any one of these risk factors: history of lobular carcinoma in situ, atypical lobular hyperplasia, atypical ductal hyperplasia, heterogeneously or extremely dense breast tissues on mammography, and women with a personal history of breast cancer, including DCIS. They recommended against MR screening, based on expert consensus opinion, in patients with a less than 15% lifetime risk of breast cancer.
Most patients who have had radiation to the chest at a young age, between 10 and 30 years, are Hodgkin lymphoma survivors. Breast cancer is the most common second malignancy among female survivors of treated Hodgkin lymphoma, with a higher risk in those treated for Hodgkin lymphoma before age 30 years, with greater than 40 Gray dose of radiation, and with mantle field radiation compared with mediastinal radiation alone. The risk of breast cancer approaches 29% in a woman aged 55 years who is treated for Hodgkin lymphoma at age 25 years. The increased risk of secondary breast cancer emerges after a latency period of 8 to 10 years after treatment. The mammographic and sonographic appearances are similar to sporadic breast cancer; however, the malignancies tend to have unfavorable pathologic characteristics and are invasive cancers more often than DCIS. Dense breast tissues may also limit the sensitivity of screening mammography in young premenopausal women such as Hodgkin survivors. Mammography may be effective at detecting DCIS, but may be inadequate for detection of invasive breast cancer in this high-risk population. Based on expert consensus opinion, the American Cancer Society recently recommended MR imaging as an adjunct to mammography in this high-risk population.
At the Dana-Farber Cancer Institute, the authors recommend annual mammography beginning age 25 and supplement screening with MR in patients with known or suspected BRCA or familial mutations. In patients with Hodgkins lymphoma treated with mantle radiation, the authors screen beginning 8 years following treatment in patients age 25 or over and supplement screening with MR. If the patient does not live a great distance away and travel is not an impediment, the screening mammogram and MR are offset by 6 months so that the patient receives 1 screening study every 6 months, in the hope that the more frequent screening will detect a developing malignancy earlier.
Further investigation needs to be done in the subset of patients at 15% to 20% increased lifetime risk of breast cancer to determine which of these women benefit from screening MR. This group includes women with a personal history of breast cancer, including DCIS, lobular carcinoma in situ, atypical lobular hyperplasia, atypical ductal hyperplasia, and heterogeneously or extremely dense breast tissues on mammography.
Screening for the contralateral breast
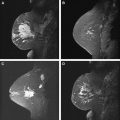
Several single-institution studies have been performed in patients with a newly diagnosed invasive breast cancer with a 3% to 5% detection rate by MR of synchronous cancers in the contralateral breast (range 3%–24%), otherwise occult on clinical breast examination and mammography. In a recent prospective multi-institutional trial of women with a newly diagnosed breast cancer, the American College of Radiology Imaging Network (ACRIN 6667) MRI Evaluation of the Contralateral Breast in Women Recently Diagnosed with Breast Cancer, MR detected 30 contralateral cancers among 969 women with a negative mammogram, at a rate of 3.1%. The sensitivity of MR was 91%, specificity was 88%, and the negative predictive value was 99%. The biopsy rate was 12.5% (121/969), finding 30 cancers, of which 18 were invasive. The mean size of the cancers was 10.9 mm. All were small cancers with a favorable prognosis. No patients had distant metastases, and of the patients with node status known (27/30), none had positive lymph nodes. This result suggests that occult contralateral malignancies are detected by MR at an early stage that is treatable. Fig. 3 shows a patient in whom MR detected more extensive malignancy than was originally suspected, and an occult contralateral cancer.

If a contralateral suspicious lesion is detected at MR, it is essential that biopsy diagnosis be established before surgical planning, as not all suspicious lesions are malignant.
Screening for the contralateral breast
Several single-institution studies have been performed in patients with a newly diagnosed invasive breast cancer with a 3% to 5% detection rate by MR of synchronous cancers in the contralateral breast (range 3%–24%), otherwise occult on clinical breast examination and mammography. In a recent prospective multi-institutional trial of women with a newly diagnosed breast cancer, the American College of Radiology Imaging Network (ACRIN 6667) MRI Evaluation of the Contralateral Breast in Women Recently Diagnosed with Breast Cancer, MR detected 30 contralateral cancers among 969 women with a negative mammogram, at a rate of 3.1%. The sensitivity of MR was 91%, specificity was 88%, and the negative predictive value was 99%. The biopsy rate was 12.5% (121/969), finding 30 cancers, of which 18 were invasive. The mean size of the cancers was 10.9 mm. All were small cancers with a favorable prognosis. No patients had distant metastases, and of the patients with node status known (27/30), none had positive lymph nodes. This result suggests that occult contralateral malignancies are detected by MR at an early stage that is treatable. Fig. 3 shows a patient in whom MR detected more extensive malignancy than was originally suspected, and an occult contralateral cancer.
If a contralateral suspicious lesion is detected at MR, it is essential that biopsy diagnosis be established before surgical planning, as not all suspicious lesions are malignant.
Implants and breast augmentation
Screening MR may be helpful in patients with augmentation or cosmetic injections. MR has been shown to better visualize tissue around a silicone implant. Detection of malignancy may be challenging with mammography in patients with free injection of materials such as silicone, paraffin, or polyacrylamide gel; MR in these cases may detect malignancy to better advantage. MR may also detect malignancies that occur posterior to an implant, which would not be visualized mammographically because of the posterior location.
MR imaging has been shown to have the highest sensitivity and specificity for the diagnosis of silicone implant rupture without the use ionizing radiation. Sensitivity ranges from 28% to 59% for mammography, and 78% to 81% for MR. To optimize detection of implant rupture, no contrast is given to the patient- and silicone-selective sequences, and water-suppression techniques are used. If the clinical indication is to evaluate for possible implant rupture, the technique is not designed to evaluate for breast cancer, as no contrast is given to the patient and the sequences are not optimized for breast cancer detection.
When an implant is surgically placed, the body forms a fibrous capsule of scar tissue around the implant. In patients with a saline implant, a rupture will be clinically evident, with a decrease in the size and contour of the breast, and no imaging will be necessary. In patients with a silicone implant, the implant may be intact, or there may be an intracapsular or an extracapsular rupture. Most silicone implants more than 10 years old have a rupture, most commonly intracapsular. An intracapsular rupture occurs when the envelope breaks, but the silicone remains contained by the fibrous capsule. This may be seen as a linguine sign on MR imaging. Signs of an incomplete envelope collapse on MR are the subcapsular line sign, teardrop sign, and keyhole sign.
An extracapsular rupture represents extrusion of silicone outside the fibrous capsule. On breast MR, silicone may be seen within the breast tissues outside the implant or in the axillary lymph nodes. The signal intensities on the MR sequences obtained will follow silicone.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree