Dynamic contrast-enhanced magnetic resonance (MR) imaging of the breast is a useful tool for the assessment of both structural and functional anatomy. A basic approach to the interpretation of normal anatomy on breast MR imaging is reviewed in this article.
Breast cancer is one of the most common cancers in American women, with the American Cancer Society estimating more than 200,000 women being newly diagnosed with breast cancer in 2010. As an adjunct to mammography, breast magnetic resonance (MR) imaging has emerged as a powerful and useful tool in the detection and evaluation of breast cancer. Dynamic contrast-enhanced breast MR has sensitivities reported as approaching 100% for invasive breast cancer. Given this sensitivity, breast MR imaging is increasingly being used in both screening and diagnostic breast imaging applications. In 2007, the American Cancer Society published guidelines for the application of screening breast MR imaging in women with an elevated risk of breast cancer, either by family history–based calculation or because of prior medical history. The use of breast MR imaging in specific diagnostic scenarios such as evaluating the extent of disease in a patient with breast cancer has also been studied. As the applications of breast MR imaging increase and are refined, an understanding of the normal anatomy of the breast on MR imaging and the basic rationale behind the imaging of this structure is invaluable. Only with a strong foundation of knowledge of the “normal” can pathology be recognized appropriately.
The goals of this article are as follows:
- 1.
Review a basic protocol for breast MR imaging
- 2.
Review normal structural anatomy of the breast
- 3.
Review expected range of normal functional anatomy of the breast, as seen on dynamic contrast-enhanced images of the breast.
Protocol: technical considerations
Breast MR imaging has the ability to depict both structural and functional anatomy of the breast. Breast MR imaging protocol uses a multiplanar imaging approach as well as the use of intravenous gadolinium to achieve the goals of anatomic depiction. This discussion focuses on the protocol currently used at the Hospital of the University of Pennsylvania (HUP), with reference to general principles of breast MR imaging techniques.
At present, the standard clinical protocol for breast MR imaging at this institution is acquired with a 1.5-T magnet with a dedicated bilateral breast coil. A minimum field strength of 1.5 T is recommended for optimal breast MR imaging. It is important that there is good magnetic field homogeneity across both breasts, to allow for optimal in-plane image resolution and signal to noise ratio per pixel. Also, this allows for optimal fat suppression across both breasts.
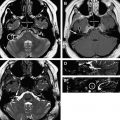
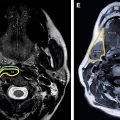
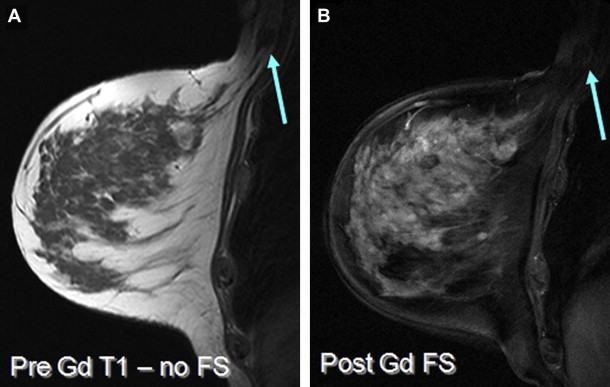
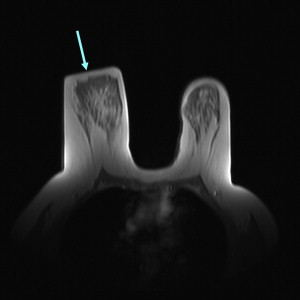
The importance of patient positioning should be emphasized. For breast MR imaging, the patient is positioned prone with the breasts pendant. It is essential that the technologist positioning the patient is aware of the pitfalls sometimes associated with use of the breast coil and prone positioning. For example, lateral and axillary breast tissue is often excluded from the coil, and this is sometimes only apparent during localizer sequences ( Fig. 1 ). This situation can lead to nonvisualization or suboptimal evaluation of the excluded portions of the breast. Such malpositioning may limit the visualization of not only the structural anatomy of those portions of the breast(s). It must be noted that the pressure from the patient’s weight on the portions of the breast excluded from the coil also alters the vascular flow dynamics of those areas. Thus, the functional anatomy of those portions of the breast is also not optimally evaluated, which can lead to false-negative results ( Fig. 2 ).
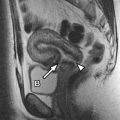
Positioning is also of paramount importance during MR imaging–guided breast interventional procedures. Although this topic is beyond the scope of this article, it is important to be aware of the impact of positioning on the potential success of any MR imaging–guided breast procedure. Consideration should be given to physical access to the target site and to the impact of altering the enhancement dynamics to the target site during such procedures.
The patient’s arms are variably positioned either above the head or at the sides, depending on patient mobility, patient comfort, and ability to image or access the target region of the breast. The patient’s peripheral intravenous access is connected to the injector before imaging, to minimize any subsequent patient motion.
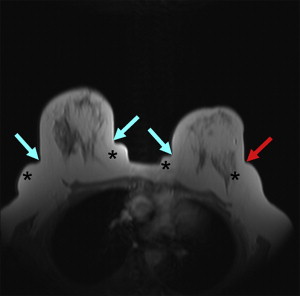
Localizer sequences are first performed ( Table 1 ). In addition, patient positioning can also be assessed on localizer sequences. If necessary, the patient should be repositioned (see Fig. 1 ; Fig. 3 ).
| Parameter | Imaging Series | ||||
|---|---|---|---|---|---|
| Multiplanar Localizer | Sagittal T2-Weighted | Sagittal T1-Weighted GRE | Sagittal T1-Weighted GRE (pre- and 3 postcontrast series at 90-s intervals) | Axial T1-Weighted GRE | |
| Concatenations | — | 2 | 2 | 1 | 1 |
| TR (ms) | 20.0 | 6530 | 9.76 | 14.6 | 8.13 |
| TE (ms) | 5.00 | 86 | 4.76 | 3.61 | 3.83 |
| Fat saturation | No | Yes | No | Yes | Yes |
| Flip angle | 40 | 180 | 20 | 30 | 15 |
| Field of view (mm) | 400 | 240 | 240 | 240 | 320 |
| Matrix | 256 | 256 × 256 | 512 × 512 | 512 × 512 | 512 × 512 |
| No. of sections | 1 each plane | 58 | 88 | 88 | 160 |
| Slice thickness (mm) | 10.0 | 3 | 2.9 | 2.9 | 1.5 |
| Voxel size (mm) | 3.1 × 1.6 × 10.0 | 1.3 × 0.9 × 3.0 | 0.7 × 0.5 × 2.9 | 0.7 × 0.5 × 2.9 | 0.9 × 0.6 × 1.5 |
| Bandwidth (Hz/pixel) | 180 | 129 | 180 | 280 | 200 |
| Relative SNR | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Imaging time (min:s) | 0:12 | 3:57 | 1:37 | 1:24 (×4) | 1:42 |
At the author’s institution, sagittal plane imaging is performed for all precontrast series and dynamic enhanced series. A delayed axial postcontrast series is also obtained for multiplanar reference purposes, as well as for delayed enhancement information. The ability to cross-reference between planes is useful in establishing the 3-dimensional characteristics of any finding. Sagittal image acquisition is chosen at HUP to maximize the in-plane spatial resolution of the images, thus optimizing morphologic assessment of any findings. Axial dynamic image acquisition is an alternative option, and can provide excellent temporal resolution (fewer total slices to image both breasts) and also provides the ability to compare symmetry on the same image. There are pros and cons to each approach; one compromise solution is to obtain dynamic enhanced series with isotropic voxels, so that reconstruction in the imaging plane of choice can occur with postprocessing. The essence of any breast MR imaging technique with regard to imaging plane is to ensure there is some capability of multiplanar assessment and cross-referencing, so that any enhancement can be properly assessed in terms of 3-dimensional morphology.
The phase-encoding direction is chosen to minimize artifact from cardiac pulsation. This direction will vary based on the chosen imaging plane. For example, the phase-encoding direction will be craniocaudal in the sagittal imaging plane and transverse in the axial imaging plane. Although in the axial plane this results in some cardiac pulsation artifact in the axillary regions, it is preferable to obscuring the medial portions of the breasts (which is what would occur if the phase-encoding direction were chosen in the orthogonal axis).
After localizer sequences, at HUP a sagittal T2-weighted turbo spin-echo sequence is acquired (see Table 1 ) with fat saturation ( Fig. 4 ). The T2 signal intensity of an MR imaging finding has been reported to be useful as an adjunct to morphology and kinetic information. Although there are many benign MR imaging findings that have increased T2 signal, such as cysts and fibroadenomas, it is important to remember that malignant lesions can also have increased T2 signal. One study reported that 30 of 480 cancers displayed increased T2 signal relative to surrounding parenchyma on fat-suppressed T2-weighted imaging. Increased T2 signal intensity of a finding can thus support a benign assessment if morphology and enhancement is otherwise also benign, but the increased T2 signal intensity of a lesion should not trump suspicious morphology or enhancement kinetics.