(1)
Department of Radiology, Keck School of Medicine, University of Southern California, Los Angeles, CA, USA
(2)
Keck School of Medicine, University of Southern California, Los Angeles, CA, USA
Case 3.1: Ductal Carcinoma In Situ of Breast
History
A 45-year-old female with biopsy-proven diagnosis of ductal carcinoma in situ of right breast. PET/CT was performed for initial staging.
Hypermetabolic right breast mass, SUVmax 13.8 (black arrow). Intensely hypermetabolic right axillary lymph nodes (LN), most avid LN demonstrates SUVmax 16.4 (yellow arrow).
Impression
Hypermetabolic right breast mass with at least two hypermetabolic right axillary LN, consistent with biopsy-proven malignancy.
Pearls and Pitfalls
Positron emission tomography (PET) with fluorine 18 fluorodeoxyglucose (FDG) is used to diagnose, stage, and monitor breast cancer. FDG-PET has the capability to depict abnormal metabolic activity before any anatomic change occurs. The most important advantage of FDG-PET/CT compared with other imaging modalities is the capability of detecting unsuspected distant metastases during a single whole-body examination. However, the results of most studies show that the capability of PET to depict lesions smaller than 1 cm in diameter is constrained by limited spatial resolution. PET also is of limited use for identifying tumors that are well differentiated histologically, such as ductal carcinoma in situ, and slow-growing cancers such as tubular carcinoma [1].
Discussion
DCIS is defined as proliferation of epithelial cells confined to mammary ducts. The frequency of the diagnosis of DCIS has increased markedly in the United States since the widespread use of screening mammography [2]. DCIS comprises of a heterogeneous group of histopathological lesions that have been classified into several subtypes based primarily on architectural pattern: micropapillary, papillary, solid, cribriform, and comedo. Comedo-type DCIS appears to be more aggressive, with a higher probability of associated invasive ductal carcinoma [3].
Treatment options for patients with DCIS [2]:
1.
Breast-conserving surgery and radiation therapy with or without tamoxifen
2.
Total mastectomy with or without tamoxifen
3.
Breast-conserving surgery without radiation therapy
Case 3.2: Invasive Breast Cancer
History
A 31-year-old female diagnosed with invasive right breast cancer and positive axillary metastases. She underwent modified radical mastectomy (MRM) and sentinel lymph node dissection, followed by chemotherapy.
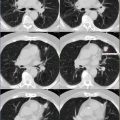
(Right breast) Irregular enhancing mass measuring approximately 1.7 × 1.3 × 2.8 cm, approximately 6 cm from the nipple, in the deep outer right breast (white arrow). No scan evidence of overlying skin, underlying muscle, or nipple involvement. (Left breast) No occult masses or abnormal areas of enhancement or adenopathy.
Hypermetabolic mediastinal lymph nodes seen best in coronal image on left (subcarinal lymph node showed SUVmax 6.6). Postsurgical changes in the right axilla. Minimally lytic, hypermetabolic lesion at L4, SUVmax 6.6 (coronal view and bottom right axial image).
Follow-up PET/CT (obtained 2 years from initial diagnosis and chemotherapy). Metabolic activity in the surgical bed of right axilla may represent chronic postsurgical changes versus residual disease. Resolution of hypermetabolic lymphadenopathy in the mediastinum. Resolution of hypermetabolic, L4 lytic lesion, now replaced by inactive, dense sclerosis, consistent with treated disease.
Impression
Interval resolution of activity within previously seen hypermetabolic mediastinal lymph nodes and L4 lytic lesion. Mild activity at the right axillary tail, likely postsurgical inflammation. No scan evidence of local recurrence.
Pearls and Pitfalls
FDG-PET is valuable for monitoring the effects of chemotherapy [4, 5]. Clinical examination and mammography are of limited use for monitoring the treatment response because of the difficulty in distinguishing fibrosis from residual tumor [1].
However, false-positive FDG uptake has been observed in some benign breast diseases, e.g., in the presence of fibrocystic change, atypical ductal hyperplasia, ductal ectasia, and phyllodes tumor [1].
Discussion
PET can demonstrate changes in tumor metabolism before morphologic changes occur; thus, unresponsive tumors can be identified quickly. The uptake of FDG in a tumor after chemotherapy is predictive of the response to therapy; a treatment-induced reduction in metabolic activity correlates with a positive clinical response [1].
Practice guidelines from the SNM, National Comprehensive Cancer Network (NCCN), and other professional groups summarized for Breast Cancer (March, 2012):
1.
Initial staging of patients with locally advanced or metastatic breast cancer when conventional staging studies (e.g., CT or bone scan) are equivocal or suspicious
2.
Follow-up or surveillance of patients with breast cancer when conventional studies (e.g., CT or bone scan) are equivocal or suspicious
Case 3.3: Osseous Metastatic Disease Secondary to Intraductal Breast Carcinoma
History
A 54-year-old female, previously diagnosed with intraductal carcinoma (IDCA) of right breast, refused therapy, and now presents with stage IV disease.
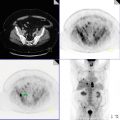
Hypermetabolic, right subareolar mass also involving skin (known primary neoplasm), SUVmax 13.0, hypermetabolic satellite lesion lateral to the primary lesion (yellow arrow), right axillary lymph nodes, hypermetabolic contralateral left internal mammary lymph node (axial PET image), hypermetabolic retroperitoneal and pelvic lymphadenopathy (coronal PET/CT images). Multiple hypermetabolic osseous lesions (coronal PET/CT images) involving C5, left scapula, sternum, T7, L3 vertebrae. Pathologic compression fracture of L5. Hypermetabolic lesion involving sacral promontory, predominantly lytic left iliac bone lesion extending to the posterior left acetabulum (red arrow).
Impression
Stage IV, metastatic breast cancer with hypermetabolic nodal disease above and below the diaphragm and multiple osseous metastases.
Pearls and Pitfalls
18F-FDG-PET/CT may be an independent prognostic factor for disease recurrence in patients with invasive ductal carcinoma [6]. Intraductal carcinoma also refers to as ductal carcinoma in situ (DCIS).
Discussion
Skeletal metastases of breast cancer mainly occur from lymphatic spread and hematogenous dissemination. A part of the sternal metastasis could occur via lymphatic vessels to parasternal lymph node. The prognosis of patients with solitary metastatic bone lesions, especially sternal metastasis, may be different from that of multiple skeletal metastasis, because sternal metastasis may be caused by local tumor invasion from either the primary site or adjacent lymph nodes [7]. Coleman et al. [8] reported that important prognostic factors for survival after the development of osseous metastasis in breast cancer depended on the histopathological grade of the primary tumor, ER status, presence of skeletal metastasis at initial breast cancer diagnosis, disease-free interval, and age.
Case 3.4: Metastatic Breast Cancer
History
A 5-year-old female with right breast cancer and hepatic metastases, on chemotherapy.
(Top row image) Hypermetabolic solitary right axillary LN, SUVmax 6.8, consistent with metastatic disease. (Middle row image) Hypermetabolic soft tissue density in the medial quadrant of the lower right breast, SUVmax 8.3, consistent with known breast primary. (Bottom row image) Large hypermetabolic left hepatic lobe lesion, SUVmax 14.0, with central necrosis.
Impression
Hypermetabolic right breast primary neoplasm with ipsilateral right axillary LN metastasis and hypermetabolic hepatic metastasis.
Pearls and Pitfalls
PET/CT may be more sensitive than serum tumor markers in detecting relapse of breast cancer [9].
Discussion
(90)Y radio embolization (selective internal radiation therapy [SIRT]) has emerged as a valuable therapeutic option in unresectable, chemotherapy-refractory hepatic metastases from breast cancer. The change in SUV (max) as assessed by (18)F-FDG-PET/CT before and 3 mos after SIRT was identified as the only independent predictor of survival in patients with hepatic metastases of breast cancer [10].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree