LEARNING OBJECTIVES
1. Equipment and scanning techniques used in breast ultrasound
2. Image labeling of breast ultrasound images
3. Technical considerations in breast ultrasound
4. Normal breast anatomy on ultrasound
5. Breast ultrasound terminology
6. Indications for breast ultrasound
7. Ultrasound features of benign lesions
8. Ultrasound features of malignant lesions
• Establishing clinical, mammographic, and ultrasound concordance
EQUIPMENT AND TECHNICAL ISSUES
Our dedicated ultrasound equipment is in immediate proximity to the mammography, stereotactic and magnetic resonance (MR) equipment. The ultrasound units are equipped with 12.5- and 17.5-MHz transducers. Optimization of the scanning parameters is critical. As discussed with mammographic image quality, it is important that you maximize ultrasound image quality. Set your quality standards high, and monitor image quality on a patient-by-patient basis. Do not settle for suboptimal equipment or images (1,2). Review and become familiar with the guidelines from the American College of Radiology regarding breast ultrasound (3). Unfortunately, and somewhat reminiscent of the experience with mammography image quality in the 1980s and early 1990s, breast ultrasound image quality at many facilities may not be what is required for good diagnostic work. On a review of 152 breast ultrasound examinations from 86 different institutions, Baker and Soo reported noncompliance with at least one of the ACR guidelines for breast ultrasound in 60.5% of studies (4). It is only when optimized equipment, image quality, and meticulous scanning technique are used that ultrasound becomes an invaluable tool in the evaluation and management of women with breast-related diseases. Anything else is often misleading and may be worse than nothing.
The number and positioning of the focal zones need to be set appropriately. The focal zone(s) should be at the level of the lesion or, at most, 1 cm superficial or deep to the anterior and posterior margins of the mass, respectively. Gain settings should enable the distinction between a simple cyst and solid mass. In the absence of a cyst, fat lobules should have varying shades of gray and not be so light as to be white or so dark as to be black. The field of view is adjusted so that an adequate amount of tissue is seen surrounding the lesion being evaluated; ideally, the images include the pectoral muscle (chest wall); however, depending on the location of the lesion and the size of the breast, this may not be achievable. It should be possible to establish the distance from the skin to the mass, and there should be enough posterior tissue to assess any changes in the transmission of the ultrasound beam beyond or deep to the lesion (2,3). Lesions can be excluded from the image if the field of view is inappropriately “zoomed” (Fig. 4.1A, B). In patients with large masses, it may be difficult to image the entire lesion and surrounding tissue using a rectangular image; a trapezoidal acquisition of the image may be used to include more of the lesion. Depending on the unit and transducer used, a standoff pad may be needed to evaluate superficial lesions. The standoff pad increases the distance from the transducer to the lesion, helping position the lesion at an appropriate depth of focus for the transducer. If the standoff pad is not used for superficial lesions, near-field artifacts may be created, leading to an inaccurate characterization of lesions. Some of the main technical considerations when doing breast ultrasounds are shown in Table 4.1.
SCANNING TECHNIQUE
Currently, breast ultrasound is an adjunctive tool used to evaluate areas of mammographic, MR, or clinical concern. Although the entire breast can be scanned, studies limited to the area of mammographic or clinical concern are common. Whole breast ultrasound units are now available on the market to facilitate automated scanning of the entire breast. For targeted ultrasound, the patient is positioned so that the tissue in the area of interest is thinned as much as possible. Supine positioning is used when evaluating the inner quadrants, and oblique positioning for tissue in the lateral quadrants and axilla; depending on breast size, the patient may be in a decubitus position to evaluate potential findings laterally. In evaluating the outer quadrants and axillae, the patient is asked to raise the ipsilateral arm and place it comfortably under her head. This helps to further thin the area being scanned. One of the major advantages of ultrasound is the real-time capability. Patients are positioned as needed to evaluate the area of concern, and physical examination is done as the transducer is rotated and varying amounts of pressure are applied over a potential or real lesion. If the patient describes an abnormality as more apparent in the upright position, she is scanned initially in an upright position. When there are significant physical limitations, patients can be scanned in a wheelchair or stretcher.

FIG. 4.1 • Field of view. A: Inappropriate field of view (FOV). The mass being followed is only partially included on the image (arrows), and image annotation has been placed over the lesion. The calipers were placed on a fatty lobule assumed to be the lesion not included with FOV. B: An appropriate FOV includes the mass (arrow) as well as the surrounding tissue preferably to include pectoral muscle. Although “zooming” on a lesion is sometimes done, in establishing the presence of a lesion, it is important to have a field of view that includes enough of the tissue to be sure that a lesion is not excluded. In most patients, if the tissue in the area of lesion has been thinned out as much as possible, the relationship to the pectoral muscle, ribs, and chest wall is demonstrated.
Table 4.1 BREAST ULTRASOUND: TECHNICAL CONSIDERATIONS
High transducer frequency (12.5 MHz, 17.5 MHz), linear array transducer |
Focal zone(s) positioning |
Gain setting |
Field of view |
Lesion in perpendicular (orthogonal) projections |
Maximal dimensions for the lesion in orthogonal projections |
Image annotations: laterality, lesion location (o’clock position, quadrant, or shown on diagram of breast), probe orientation |
Film labeling: patient’s first and last names, unique identification number, facility name, location of facility date of study, and operator’s identification |
The reason for the study and how the study is done are explained to the patient. The coupling gel is pre-warmed in commercially available warmers. If the gel is used at room temperature, it can be uncomfortably cold, particularly in the winter months. As with positioning for the mammographic study, it is important to consider the patient’s modesty. Only the breast to be examined and scanned is exposed. The contralateral breast is kept covered unless comparison needs to be made. At the completion of the study, the patient is provided a towel to wipe the coupling gel off her breast. A second person is always in the room when we scan patients.
Scanning by the radiologist is encouraged strongly. The breast imaging radiologists do all of the breast ultrasounds at our facility. We do not have an ultrasound technologist. A mammography technologist, or an assistant, helps us during diagnostic and interventional ultrasounds, but they do not scan. We have several reasons for taking this approach. First, by having the radiologist do the ultrasound studies, we are able to examine the patient and correlate what is described, or seen on the mammogram, with our own physical exam and what is imaged on the ultrasound. Real-time scanning and palpation are critical to our final impression. The information obtained during the physical examination is as important as the imaging information. Second, ultrasound is an operator-dependent study. Normal tissue can be made to simulate a lesion, and “true” lesions can be overlooked easily. To establish the presence of a lesion, the transducer needs to be rotated over the potential finding as variable amounts of pressure are applied to eliminate artifactual shadowing generated by ligaments. Third, as physical examination is done, and an impression is generated during the real-time scan portion of the study, selective images are taken to document the features of the lesion, leading us to make a specific recommendation (Table 4.2). We do not take images of normal tissue. Lastly, by doing the ultrasound ourselves, we can establish rapport with patients. We can elicit a pertinent history, reassure the patient we are doing everything possible to take care of her, and discuss findings and recommendations. The patient’s input is sought, and she is involved in the decision-making process. After discussing all reasonable alternatives, and taking clinical and imaging data into consideration, we provide patients with a specific recommendation.
Visual inspection of the breast is done with the lights up in the room prior to scanning. We specifically evaluate the breasts for changes in size and contour. We evaluate the nipple areolar complex for any erosion and nipple discharge. We establish the presence of skin findings, including skin dimpling, retraction, erythema, ecchymosis, localized thickening or thinning, peau d’orange changes (localized or generalized), eczematous changes, bullae, discoloration, or hyperpigmentation (see Figs. 8.1 through 8.7 and 9.30 for examples of findings on physical examination). In examining the breast, is the temperature of one increased compared with the contralateral side? If the patient presents with a localized finding, she is asked to pinpoint its location. If you palpate a mass, is it tender? Is it mobile or fixed? Are there any findings in the axilla? After the visual inspection and initial palpation, the lights in the room are dimmed. In further establishing the presence and physical characteristics of a mass, the transducer coupling gel is helpful in that it appears to facility and improves palpation. By combining palpation of the tissue with transducer movements, you are in essence placing an eyeball at the tips of your fingers (Fig. 4.2).
Table 4.2 FEATURES OF LESIONS TO CONSIDER ON ULTRASOUND
Malignant | Benign | Indeterminate |
Spiculation | Intensely | Isoechoic |
| hyperechoic, |
|
| homogeneous |
|
Angular margins | Oval, ellipsoid shape | Mildly hypoechoic |
Marked hypoechogenicity | Gentle bi- or trilobulation | Heterogeneous echotexture |
Shadowing | Thin echogenic pseudocapsule | Homogeneous echotexture |
Calcifications | Anechoic | Normal sound transmission |
Duct extension | No malignant features | Posterior enhancement |
Branch pattern Microlobulation Echogenic rim Vertical orientation (taller than wide) |
|
|
The transducer is moved in small increments over the area of concern to the patient or the specific area (e.g., o’clock position and expected distance from the nipple) in which you are expecting to locate a mammographically or MRI-detected abnormality. One hand holds the transducer while the index, middle, and ring fingers of the contralateral hand are placed at the leading edge of the transducer palpating the area being scanned (Fig. 4.2). If a possible abnormality is detected, the transducer is rotated 360 degrees over the area to help distinguish a mass from a fat lobule in cross section. A mass maintains a round, oval, or irregular shape as the transducer is rotated, whereas fatty tissue elongates and fuses with surrounding structures (Fig. 4.3). Varying amounts of pressure can be applied over the area being scanned and any possible abnormality detected. Traditionally, transverse and sagittal orientations are used for imaging, but because ductal structures radiate out from the nipple toward the chest wall, radial and antiradial scan orientations are recommended by some for breast ultrasound (2).
Images are not taken until a decision is made about the presence of a mass or an ongoing process (e.g., inflammatory) in the breast. Making this determination involves correlation with mammographic and clinical findings, slow movements and rotations of the transducer over the area of concern, gradations in compression, time gain compensation (TGC) curve and power setting manipulations, positioning of the focal zone(s), correct field of view, and, if indicated, use of a standoff pad. In some patients, particularly those with an inflammatory or possibly diffuse change related to trauma, scanning the contralateral breast may be helpful for comparison and assessing the patient’s normal tissue echogenicity and soft tissue planes. If during real-time scanning it is determined that a lesion is present, images are then taken to document the presence and features of the lesion. Using whatever orientations demonstrate the features of the mass best, orthogonal images are taken with and without measurements for a total of four images per lesion.

FIG. 4.2 • Breast ultrasound scanning. One hand holds the transducer, while the index, middle, and ring fingers of the contralateral hand are placed at the leading edge of the transducer palpating the area being scanned (e.g., putting an eyeball at the tip of our fingers). The transducer is rotated over the area as varying amounts of pressure are applied. The ultrasound coupling gel is helpful in that it appears to facility and possibly improves palpation.


FIG. 4.3 • Importance of transducer rotation in distinguishing pseudolesions from masses. A: Cooper ligaments create a honeycomb-like “skeletal” framework for breast tissue. Oblong bundles of glandular and fatty tissues are found within the honeycombs created by the ligaments. When the tissue is imaged longitudinally, the oblong tissue bundles are easily detected as normal tissue. When viewed in cross section, however, they can sometimes closely mimic a mass. Rotation (90 degrees) and sometimes minor rocking of the transducer over a suspected mass is critical in determining the significance of any potential finding. B: If a mass is present in the tissue bundles, it is seen three-dimensionally as the transducer is manipulated and rotated over the suspected mass. Palpation is undertaken as the tissue is scanned. (From Cardeñosa G. Breast Imaging [The Core Curriculum Series]. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.)
The study (2–4) should include the name of the patient, unique patient identifying number, date of study, name and location of facility, as well as the initials of the individual doing the ultrasound. The images need to be further annotated with respect to the breast being imaged, clock location of the lesion (Fig. 4.4A), distance of the lesion from the nipple, transducer orientation (radial/antiradial, transverse/sagittal, or longitudinal) (Fig. 4.4B), and depth in the breast (retroareolar, anterior third, middle portion, posterior third, axillary tail, axilla). Some ultrasound units provide diagrams that adequately annotate the location and scan plane used for a given image. As the transducer is manipulated, the size of the lesion varies. In providing a measurement, an attempt is made to use the largest dimensions of the lesion (2–4). If the mass demonstrates a thick echogenic rim, that rim should be included in the measurements. It is also useful to indicate on the image if the lesion is palpable. As mentioned in Chapter 3, many screen-detected abnormalities are palpable when, based on the mammographic findings, one knows exactly where to palpate.
TERMINOLOGY
Several terms are used to characterize the appearance of tissue and lesions on ultrasound. In the breast, the echogenicity of an area of interest is compared with the appearance of subcutaneous fat (2). If the area contains fewer echoes (i.e., appears darker) compared with subcutaneous fat, it is hypoechoic; if it contains more echoes (i.e., appears whiter), it is hyperechoic; if it contains no echoes (i.e., it is black), it is anechoic, and if it is of the same echogenicity as subcutaneous fat, it is isoechoic. At the junction of tissues with significant acoustic impedance differences, variable amounts of the sound beam are reflected back, and a shadow is seen. The size of the shadow depends on the size of the interface. Shadowing is a feature of some lesions, and although more common with malignant lesions, it is also seen with benign masses (Fig. 4.5A). In masses with associated calcifications, shadowing can sometimes be seen distal to the calcifications (Fig. 4.5B, C).
Simple cysts do not attenuate sound energy. Consequently, a proportionately greater amount of sound energy reaches the tissues deep to a cyst. The TGC curve, however, compensates for presumed decreases in echo strength as the distance from the transducer is increased. The resulting effect is that tissue deep to cysts appears more echogenic than adjacent tissue. This increased echogenicity deep to fluid-filled structures is called posterior acoustic enhancement or increased sound (through) transmission. Enhancement is common with cysts; however, it can be seen with benign (Fig. 4.5) and malignant masses.

FIG. 4.4 • Image labeling, ultrasound. A: Lesion location is indicated by using the o’clock position of the lesion in the breast and the distance of the lesion from the nipple. B: Images are obtained in orthogonal projections. This includes (1) radial (rad) and antiradial (arad) or (2) transverse (trv) and sagittal or longitudinal (sag, long). (From Cardeñosa G. Breast Imaging [The Core Curriculum Series]. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.)
FIG. 4.5 • Hyalinized fibroadenomas; multiple peripheral papillomas. A: An irregular, lobulated hypoechoic mass with shadowing is imaged. No calcifications are seen mammographically. Shadowing is more commonly associated with malignant masses; however, it can be seen with benign lesions, including hyalinized fibroadenomas, focal fibrosis, and masses with associated calcifications. BI-RADS 2: Benign finding. B: Mediolateral oblique (MLO) view photographically coned to the upper portion of the image in a different patient. Two low-density oval masses (arrows) are new compared with the study done a year ago. A coarse calcification is noted associated with the smaller of the two masses. C: Two adjacent masses (arrows) are seen on ultrasound corresponding to the masses seen on the mammogram. The larger of the two is oval with circumscribed margins and posterior acoustic enhancement. The smaller mass contains internal specular echoes and associated shadowing reflecting the presence of the calcifications seen on the mammogram. BI-RADS 4A: Suspicious abnormality, biopsy is indicated. Papillomas are diagnosed on core biopsy and confirmed on excisional biopsy. (From Cardeñosa G. Breast Imaging [The Core Curriculum Series]. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.
Please see Appendix A (Table A.2) for the ACR BI-RADS® ultrasound lexicon. This provides the terminology to be used, and what needs to be described in reports, with respect to ultrasound findings. You should familiarize yourself with this and apply it consistently when describing breast ultrasound findings.
BREAST ANATOMY ON ULTRASOUND
Understanding the anatomy of the breast and its surroundings on ultrasound is helpful in evaluating potential lesions and their sites of origin (see Figs. 8.57 and 11.22). The pleural reflection, ribs, and pectoral muscles are deep to breast tissue and usually imaged during scanning. The pleural reflection is a hyperechoic band deep to the ribs. The tissue air interface usually leads to shadowing deep to the pleural reflection. Simulating lesions, ribs in cross section are circumscribed oval hypoechoic structures (Fig. 4.6A). Recognizing the relationship of the ribs to the pleural reflection and overlying pectoral muscles is important in distinguishing a rib in cross section from a lesion. Longitudinally, ribs are echogenic bands of variable thickness with shadowing (Fig. 4.6B). The pectoral muscles are hypoechoic with associated specular echoes that may be seen as bright spots in the muscle when in cross-section or echogenic, parallel hyperechoic bands when imaged longitudinally (Fig. 4.6C). The deep pectoral fascia is an echogenic line on the surface of the pectoral muscle that separates the muscle from overlying breast tissue.
Cooper ligaments connect deep and superficial pectoral fascial layers, thereby providing a honeycomb-like structure or a “skeleton” for breast tissue. The ligaments are hyperechoic bands that crisscross the breast, isolating oval and oblong-shaped areas of glandular and fatty tissues. Superficially, Cooper ligaments extend to the superficial pectoral fascia in the deep dermis. Given the oval and oblong shape of breast tissue bundles, transducer movements and rotation over an area of concern are important. When breast tissue bundles are imaged in cross section as round or oval structures, they can sometimes simulate a lesion. As the transducer is rotated 90 degrees, breast tissue bundles elongate and fuse with surrounding tissue; a mass maintains its round or oval shape (Fig. 4.2). Fatty breast tissue bundles also commonly have small hyperechoic bands within them not typically seen in solid masses (Fig. 4.7).

FIG. 4.6 • Normal breast anatomy on ultrasound. A: Ribs (R) in cross section simulate oval hypoechoic masses; these are often associated with shadowing (s). The echogenic line deep to the ribs (short arrows) is the pleural reflection. Two parallel echogenic lines are present in the body of the pectoral muscle, directly over the ribs. The echogenic line on the superficial surface of the muscle is the deep pectoral fascia (long arrow) and serves as the demarcation between breast tissue and chest wall structures. In this area, the pectoral muscle is thin, and there is not much overlying breast tissue. B: Different patient. Longitudinally oriented rib (R) with associated shadowing. The pleural reflection is imaged as an echogenic line (short arrow) deep to the pectoral muscle. In this orientation, round and oval specular echoes are imaged in the pectoral muscle. The echogenic line (long arrows) on the surface of the muscle is the deep pectoral fascia. A small amount of overlying breast tissue is seen on this image. C: The specular echoes noted in the muscle on the image in part B elongate and become parallel echogenic lines in the substance of the muscle as the transducer is rotated 90 degrees. The pleural reflection is again seen as an echogenic line deep to the muscle; a portion of rib (R) with associated shadowing is also noted. The echogenic line associated with the surface of the muscle is the deep pectoral fascia (arrows) and separates the muscle from overlying breast tissue. (From Cardeñosa G. Breast Imaging [The Core Curriculum Series]. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.)
Dense glandular tissue is relatively hyperechoic (Fig. 4.8). Since most breast lesions are hypoechoic relative to subcutaneous fat, lesions are identified more readily in women with dense tissue mammographically. Cooper ligaments are not as apparent in hyperechoic fibrous tissue because they are isoechoic with the fibrous tissue. In women with predominantly fatty tissue, however, lesions may be isoechoic with surrounding tissue and not identifiable. In fatty tissue, Cooper ligaments are identified more easily because they are hyperechoic relative to the surrounding tissue (Fig. 4.7). In this patient population, however, mammography is reliable in depicting small water-density lesions. During pregnancy, breast tissue usually becomes more homogeneous with small round and oval anechoic spaces, some of which are vessels (determined with Doppler). Cooper ligaments, fibrous ridges, and tissue bundles are not as readily apparent (Fig. 4.9). Prominent ductal structures may be seen particularly in the subareolar area.
Use of the standoff pad may be required for evaluation of the skin. Normally, skin measures 1 to 2 mm and is made up of a hypoechoic band sandwiched between two hyperechoic lines (Fig. 4.10). Benign masses involving the skin (e.g., sebaceous cysts) can be localized (Fig. 4.10), and their track to the skin surface can sometimes be identified (Fig. 4.11). Edema resulting from radiation therapy acutely, congestive heart failure, benign inflammatory processes, or inflammatory carcinoma can present with skin thickening. The deep hyperechoic line may be disrupted, and there is thickening of the hypoechoic band. Small, anastomosing, serpiginous tubular structures, representing dilated lymphatics or vascular structures, are sometimes seen deep to the thickened skin (Fig. 4.12; also see Fig. 9.5B, C). Malignant and inflammatory processes arising in breast tissue can extend to involve the skin with disruption of the deep hyperechoic stripe and expansion of the hypoechoic band (Fig. 4.13; also see Fig. 9.14B). Rarely, metastatic disease to the skin can be imaged as thickening of the hypoechoic band with disruption of the superficial echogenic line when there is associated skin ulceration (Fig. 4.14).

FIG. 4.7 • Fatty tissue and lobulation. A: Round and oval mass-like areas (arrows) of fatty lobulation can be mistaken for lesions on a single static image. B: As the transducer is rotated 90 degrees, these areas fuse and elongate with surrounding tissue consistent with normal fatty lobulation. A true mass will maintain its three-dimensional shape as the transducer is rotated. (From Cardeñosa G. Breast Imaging [The Core Curriculum Series]. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.)
The nipple contains connective tissue and smooth muscle. In some women, it can produce intense shadowing, and in others it can be made to simulate a solid mass (Fig. 4.15A, B). In many patients, the transducer needs to be angled around the nipple to evaluate the subareolar area adequately. Ductal structures can be seen in the subareolar area coursing toward the nipple (Fig. 4.16). These are most commonly tubular in appearance and anechoic. In cross section, they may look more like an anechoic mass (e.g., a cyst). Rarely, they can be homogeneously hyperechoic in appearance (see Fig. 9.32B). Intraductal lesions such as papillomas can sometimes be identified, particularly if the lesion is in the subareolar portion of the duct and the duct is dilated (Fig. 4.17). However, keep in mind that ductal structures often come into and out of the scan plane, potentially causing misleading interpretations regarding the presence or absence of an intraductal mass. Radial scanning may be helpful in demonstrating the ducts coursing for variable distances away from the nipple. As the distance from the nipple increases, the likelihood of identifying intraductal lesions decreases unless the duct remains dilated.

FIG. 4.8 • Dense tissue. Dense tissue is relatively hyperechoic on ultrasound (large arrows). Visualization of Cooper ligaments is limited in this type of tissue. Small hypoechoic round and oval areas are seen in the echogenic fibrous tissue, likely reflecting ducts and lobular units. Relatively hypoechoic fatty tissue is seen superficially (small arrows). Ligaments are more easily seen in fatty tissue as echogenic lines. Muscle (M) is seen deep to the glandular tissue. (From Cardeñosa G. Breast Imaging [The Core Curriculum Series]. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.)

FIG. 4.9 • Lactational changes. During late pregnancy and lactation, breast tissue is more homogeneously hyperechoic, and demarcation of tissue bundles is less apparent. Mild-to-moderately dilated ducts with a somewhat beaded appearance can be seen coursing through the tissue for variable distances from the nipple. These can be more or less distended depending on when the last breast feeding or pumping occurred. (From Cardeñosa G. Breast Imaging [The Core Curriculum Series]. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.)

FIG. 4.10 • Skin and developing skin lesion. Spacer used to evaluate a superficial palpable mass (black arrow). Normal skin is 1 to 2 mm thick and is characterized by a hypoechoic band sandwiched between two echogenic lines. The superficial (small white arrows) and deep echogenic lines (big white arrows) are well seen in this patient. The palpable mass arises in the hypoechoic band, disrupts the deep echogenic line, and extends minimally into the subcutaneous fat of the breast. (From Cardeñosa G. Breast Imaging [The Core Curriculum Series]. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.)

FIG. 4.11 • Sebaceous cyst. On physical examination, a punctum is seen on the skin, and a mass that moves with the skin is palpated. Using the standoff pad, a vertically oriented hypoechoic mass (calipers) with circumscribed margins, posterior acoustic enhancement, and a track (arrow) to the skin is imaged on ultrasound corresponding to the palpable finding and a round isodense mass with circumscribed margins seen on the mammogram (not shown). The patient is reassured of the benign nature of the finding. This requires no additional intervention or short-term follow-up unless it becomes infected or painful, in which case surgical excision may be needed. BI-RADS 2: Benign finding.

FIG. 4.12 • Skin thickening and dilated subcutaneous lymphatics in a patient with congestive heart failure. Skin thickening is noted in this patient with congestive heart failure. The superficial and deep echogenic lines are not apparent, and the hypoechoic band is thickened. Tubular, anastomosing, serpiginous structures deep to the skin are dilated lymphatics; rarely, some of these represent vascular structures and can be characterized as such with Doppler (see Fig. 9.5B). The tissue is echogenic, and normal tissue planes are disrupted related to edematous changes. (From Cardeñosa G. Breast Imaging [The Core Curriculum Series]. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.)
Vascular structures can sometimes be imaged in cross section, less commonly longitudinally (Fig. 4.18), and are more likely to be identified in patients with ongoing inflammatory or edematous changes in the breasts (see Fig. 9.5B). Vessels are often anechoic simulating small cysts; however, pulsations are apparent if the transducer is held steady over the anechoic structure. Doppler is helpful in establishing the nature of the finding. In women with extensive, dense vascular calcification, the calcified vessels can sometimes be seen on ultrasound (Fig. 4.19). In patients presenting with Mondor disease (see Chapter 9), the dilated thrombosed vein is often imaged subcutaneously (Fig. 4.20; also see Figs. 9.39 and 9.40); no flow is seen in the vein.

FIG. 4.13 • Invasive ductal carcinoma, not otherwise specified secondarily involving the skin. A mass with shadowing is imaged disrupting the echogenic deep dermal line (large arrows). It focally thickens and extends into the hypoechoic layer (thin arrows) of the skin (also see Fig. 9.14B).

FIG. 4.14 • Leiomyosarcoma, metastatic to skin. A: MLO view photographically coned to the superior aspect of the right breast in a patient presenting with an ulcerating mass. The patient’s history is notable for previous treatment of a leiomyosarcoma. The metallic BB denotes the site of the palpable finding. Air (lucency) pocket (arrows) outlines the ulcerative portion of the mass. Vascular calcification is present. B: Ultrasound with standoff pad. A mass involves the hypoechoic band of the skin extending and disrupting the superficial (arrows) and deep echogenic lines of the skin. (From Cardeñosa G. Breast Imaging [The Core Curriculum Series]. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.)
On ultrasound, intramammary and axillary lymph nodes are characterized by the presence of a hypoechoic cortical region that partially or completely surrounds an eccentrically or centrally located hyperechoic hilar region (Fig. 4.21). Bulging and thickening of a markedly hypoechoic cortical region, and attenuation (mass effect) or complete loss of the hyperechoic fatty hilar region are nonspecific findings that may be related to metastatic disease, lymphoma, or, less commonly, benign reactive changes (Fig. 4.22). In general, the echogenicity of the cortex, even when prominent and bulging, is helpful in assessing benign from malignant lymph nodes. Benign processes involving the lymph nodes may thicken the cortex; however, the echogenicity of the cortex is often characterized as iso- to slightly hyperechoic (Fig. 4.22A). In contrast, marked hypoechogenicity of the cortex (close to anechoic) is more suggestive of metastatic disease (Fig. 4.22B). Please see Chapters 7 and 8 for more on lymph nodes.

FIG. 4.15 • Nipple. A: In many women, the nipple (N) produces an intense shadow that limits evaluation of the subareolar area and may be mistaken for a malignant lesion on a static image. B: In others, if generous amounts of coupling gel are put over the nipple and some pressure is applied, the nipple simulates an oval hypoechoic mass (arrows) that may be mistaken for a lesion on a static image. During real-time scanning, this can easily be identified as the nipple and not a mass in the subareolar area. To evaluate the subareolar area adequately, the transducer needs to be placed at the base of the nipple and angled toward the subareolar area.

FIG. 4.16 • Ductal structures in the subareolar area. A: Several ducts are imaged coursing proximally from the nipple. Since ducts and their branches course in and out of the plane of the transducer, it can be difficult to follow and image them. B: Different patient. A duct (arrows) with focal areas of dilatation can be followed from the nipple proximally. During real time it could be connected to some of the tubular structures in this image that appear separate from the main duct. This is common since ducts course in and out of the scan plane. (From Cardeñosa G. Breast Imaging [The Core Curriculum Series]. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.)

FIG. 4.17 • Papilloma in the subareolar portions of duct. Two intraductal lobulated masses (arrows) with circumscribed margins and posterior acoustic enhancement are imaged in a dilated duct in the subareolar area. Shadowing is noted associated with the nipple (N). To evaluate the subareolar area adequately, the transducer needs to be placed at the base of the nipple and angled toward the subareolar area. (From Cardeñosa G. Breast Imaging [The Core Curriculum Series]. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.)


FIG. 4.18 • Vascular structures. A: Artery in cross-section with Doppler on. These are imaged as anechoic round structures that can simulate a cyst when imaged in cross section. As the transducer is manipulated, they can sometimes be seen longitudinally. While holding the transducer over the anechoic structures, pulsations are evident during real-time scanning. Doppler is helpful in confirming these as vascular structures. B: As the transducer is rotated over the round mass, the tubular nature of the structure, and with Doppler, the vascular etiology is established. (From Cardeñosa G. Breast Imaging [The Core Curriculum Series]. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.)
INDICATIONS FOR ULTRASOUND
For many years, ultrasound was used sparingly and almost exclusively to characterize breast masses as cystic or solid. While this remains an important use, improvements in equipment have led to increases in the indications for breast ultrasound. It is now an indispensable tool in evaluating women with imaging (mammography, magnetic resonance imaging [MRI]) or clinically detected abnormalities (2). The indications include (i) Matrix characterization of palpable or mammographically detected masses; (ii) Characterization of solid masses as indeterminate or likely benign or malignant; (iii) Evaluation of nonspecific mammographic findings (e.g., developing parenchymal asymmetry, possible area of distortion); (iv) Evaluation of tissue or lesions potentially excluded on routine mammographic views (e.g., upper inner quadrants, axillary tissue, etc.); (v) Evaluation of MRI-detected abnormalities; (vi) Evaluation of women with inflammatory symptoms to distinguish mastitis from an abscess or a locally advanced breast cancer from inflammatory carcinoma; (vii) In conjunction with ductography in the evaluation of women presenting with spontaneous nipple discharge (see Chapter 12); (viii) To guide interventional procedures (Chapter 12); and (ix) For specimen evaluation (specimen sonography, see Chapter 12).

FIG. 4.19 • Vascular calcification. A: Craniocaudal (CC) views in a 77-year-old patient. Dense tissue. Extensive vascular calcification is present bilaterally. B: Ultrasound. Irregular, linear areas of echogenicity (arrows) can be seen rarely when the vascular calcification is dense and extensive as in this patient. Shadowing is seen associated with some of the calcifications. Longitudinally oriented ribs (R) are seen. (From Cardeñosa G. Breast Imaging [The Core Curriculum Series]. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.)

FIG. 4.20 • Mondor disease. A 32-year-old patient presents describing a linear area of dimpling in the right breast associated with tenderness. A tubular structure is imaged subcutaneously along the linear dimpling. No flow is seen in the acute phase of Mondor disease. With spontaneous resolution of the thrombophlebitis and recannulation of the vessel, this structure is no longer apparent 8 weeks later. (From Cardeñosa G. Breast Imaging [The Core Curriculum Series]. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.)

FIG. 4.21 • Intramammary lymph node. Oval mass (arrow) with a hypoechoic cortex almost completely surrounding (“hugging”) the hyperechoic fatty hilar region is imaged in the parenchyma. (From Cardeñosa G. Breast Imaging [The Core Curriculum Series]. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.)
The use of ultrasound for screening purposes is controversial but increasing in use. As ultrasound units, in particular whole breast ultrasound units, improve and we continue to learn, a role for screening women with dense tissue, particularly those who are at high risk for cancer development, is likely to become more common (5–11). Kolb and colleagues (6) reported a 42% increase in the detection of breast cancer with screening ultrasound in women with dense tissue mammographically. In this study, the cancers detected with screening ultrasound were similar in size and stage to those detected with screening mammography. False-positive screening ultrasound studies were reported as 2.4% by these investigators (6).
Screening ultrasound in women with predominantly fatty tissue adds little and may be misleading. In these patients, however, mammography is an excellent screening tool with a sensitivity of 98% (6). The most likely and appropriate role for screening ultrasound would be to evaluate women, particularly those with increased breast cancer risk, who have dense tissue mammographically in whom we know that breast cancers are missed on mammography; the sensitivity of mammography in women with dense tissue may be as low as 48% (6–10). Limitations of ultrasound, and arguments against its routine use for screening, relate to the inability to reliably detect calcifications, false-positive studies (6,10), and the operator dependence of the study (something that may be overcome with the use of automated whole breast units). As with mammography, quality issues and standardization of techniques and equipment would seem to be appropriate before widespread use. Also, it should be made clear to patients, referring physicians, and third-party payors that screening ultrasound would be used in conjunction with mammography and not as a replacement.

FIG. 4.22 • Abnormal, lymph nodes. A: Ultrasound done to evaluate a new dense round mass with circumscribed margins noted in the left axilla on a screening mammogram (not shown) in a 53-year-old woman with a history of sarcoid; she has been symptom-free for several years. A lymph node with circumscribed margins and a prominent, gently lobulated, slightly hyperechoic cortical region is imaged in the axilla corresponding to the dominant mass seen mammographically. Although no fatty hilum is identified mammographically, a fatty hilum is readily apparent on ultrasound, confirming the mass as a lymph node. There is no apparent mass effect on the hilum. Other smaller lymph nodes (not shown) with similar ultrasound features are imaged bilaterally. The echogenicity of the cortex and the presence of a prominent fatty suggest a benign process, particularly in a patient with a history of sarcoid; however, given the change noted mammographically, a biopsy is done. BI-RADS 4A: Suspicious abnormality, biopsy is indicated. A portion of a lymph node with numerous non-necrotizing granulomas and fibrosis without evidence of infection is reported histologically; the findings are consistent with sarcoid. B: Ultrasound in a different patient. A lymph node with a markedly hypoechoic thickened and bulging cortex and posterior acoustic enhancement is imaged in the left axilla. The fatty hilum is attenuated and eccentric in position. Compare the echogenicity of the cortex in this patient with that of the lymph node in the patient shown in part A. BI-RADS 4C: Suspicious abnormality, biopsy is indicated. Metastatic disease is diagnosed on a core biopsy.
MATRIX DETERMINATION
One of the most important roles of breast ultrasound is to help establish whether a clinical apparent mass is real or dense fibrous tissue. If the clinical or mammographically apparent mass is real, is it cystic or solid? Breast ultrasound is 96% to 100% accurate in the diagnosis of cysts when appropriate criteria are used (2,12). Simple cysts are anechoic masses, with circumscribed margins, sharp anterior and posterior walls, thin-edge shadows, posterior acoustic enhancement (Fig. 4.23; also see Fig. 7.44), and compressibility (13). Slight movements or changes in the orientation of the transducer may be needed to demonstrate posterior acoustic enhancement. Also, with small (approximately <5 mm) or deep cysts, posterior acoustic enhancement may not be demonstrable. Unless the cyst is under tension, pressure applied with the transducer leads to compression and elongation of the cyst. Reverberation artifacts can involve the anterior wall of some cysts (Fig. 4.24). If the criteria for a simple cyst are fulfilled, and the patient is asymptomatic, we do not recommend aspiration. We try to educate and reassure the patient that cysts are common, benign masses that fluctuate in size and tenderness with the menstrual cycle, may regress spontaneously, and often recur if aspirated. If the patient is symptomatic, aspiration is undertaken. Likewise, if we are not sure whether the finding is a cyst because it is complicated in appearance on the ultrasound, aspiration and sometimes pneumocystography are done (see Chapter 12).
In the early phases of cyst formation, as acini begin to distend with fluid, tightly clustered, round, 1- to 2-mm sized anechoic areas are seen on ultrasound. The clustered microcysts are separated by thin echogenic septations (Fig. 4.25; also see Fig. 7.47). Posterior acoustic enhancement may be present. Although initially described as apocrine-lined cysts (13,14), histologically these areas are commonly a combination of small cystic spaces lined with either epithelial cells or cells characterized by apocrine metaplasia (see Fig. 7.48). As the acini continue to distend with fluid, clusters of small cysts may be seen. As these small cysts coalesce, the more characteristic appearance of the “single” cyst is seen.

FIG. 4.23 • Simple cysts. Two adjacent simple cysts. Anechoic masses with circumscribed margins, thin edge shadows (arrows), sharp anterior and posterior walls, and posterior acoustic enhancement. The enhancement is seen best in the larger of the two cysts. (From Cardeñosa G. Breast Imaging [The Core Curriculum Series]. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.)

FIG. 4.24 • Simple cyst, reverberation artifact. Oval, anechoic mass with circumscribed margins, thin-edge shadows (black arrow), and enhancement. Echoes in the superficial aspect of the cyst (white arrows) reflect reverberation artifact. (From Cardeñosa G. Breast Imaging [The Core Curriculum Series]. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.)
The presence of internal echoes that move in the fluid (Fig. 4.26; see also Fig. 7.46) during real-time scanning (“gurgling,” swirling) and septations are relatively common variants (2,13). Nondependent, nongurgling internal echoes that partially or completely fill the cyst (Fig. 4.27) and irregular or thickened walls when inflamed may represent “complicated” cysts. If not all of the criteria for the diagnosis of a cyst are present, or the diagnosis of a cyst is otherwise in question, aspiration is undertaken. Cysts should collapse completely, and no residual abnormality should be seen post aspiration. If an intracystic or mural lesion is suspected, pneumocystography or a biopsy of the residual solid component can be done (see Chapter 12). Cyst fluid is not routinely submitted for cytologic evaluation unless the fluid is bloody.
Galactoceles are cysts that contain milky fluid (13). They present in women during lactation, but may be diagnosed several years following the cessation of breast-feeding. Ultrasound findings in women with galactoceles are variable. They are often indistinguishable from simple cysts, but they can be associated with atypical features, including solid appearance, complex cystic mass, or a mass with significant shadowing (Fig. 4.28; also see Fig. 7.56).
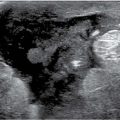
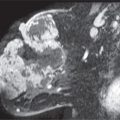
Complex cystic and solid masses can be seen in a variety of circumstances (see Chapter 7 and 8 for more complete discussions) (13). Although there can be significant overlap in the diagnostic considerations, we characterize these masses as predominantly cystic with solid components or predominantly solid with cystic components (Table 4.3). Included in the first group are postoperative or traumatic fluid collections. Following surgery, particularly in women undergoing a lumpectomy and radiation therapy or trauma, fluid collections may develop. In some patients these may appear indistinguishable from a cyst. In other patients, these fluid collections have a variable ultrasound appearance, including mural nodules, intracystic septations, or a combination of these. The walls may be irregular, lobulated, and thickened (Fig. 4.29; also see Fig. 11.26). It is important to recognize the benign nature of these collections. Unless an infection is suspected, these should be left alone. Aspiration often leads to re-accumulation of fluid, and rarely, aspiration may lead to the development of draining sinuses that can be difficult to manage (Fig. 4.30; also see Fig. 11.27). Papillomas are commonly imaged as intraductal lesions (Fig. 4.18), particularly if solitary, or as intracystic mass(es) of variable sizes (Figs. 4.31 and 4.32). In some women, they may be solid or predominantly solid with cystic components (Fig. 4.33). Oil cysts and evolving areas of fat necrosis may have stages characterized on ultrasound as a cystic lesion with mural and solid-appearing intracystic nodules (Fig. 4.34; also see Fig. 7.24). Papillary carcinomas presenting centrally as solitary lesions are often characterized as a complex cystic and solid mass (see Chapter 8).
FIG. 4.25 • Clustered microcysts. A: A cluster of small, round and oval anechoic masses. The thin walls of the distending acini are evident (arrows). BI-RADS 2: Benign finding. Although not usually indicated, if a core biopsy is done, these lesions tend to disappear after the first or second pass of the needle. Epithelial- and apocrine-lined cysts are reported histologically. B: Different patient. Spot compression view of a new finding on screening mammography demonstrates a lobulated mass with partially circumscribed and indistinct margins (thin arrow). Incidentally noted is nail polish artifact (thick arrows). C: Ultrasound image corresponding to the area of mammographic concern demonstrating a cluster of oval anechoic masses. The thin walls of the distending acini are evident as thin echogenic lines separating the anechoic components. Epithelial and apocrine-lined cysts are reported histologically. As we have acquired experience, and with our medical audit data to support our approach, we no longer routinely biopsy patients with lesions having these mammographic and ultrasound features. (From Cardeñosa G. Breast Imaging [The Core Curriculum Series]. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.)

FIG. 4.26 • Gurgling cyst (“complicated”). Specular echoes (few marked with arrows) in otherwise anechoic mass with circumscribed margins and posterior acoustic enhancement. During real-time scanning, the echoes can be seen moving and sometimes swirling in the fluid. Unless the patient is symptomatic, or requests an aspiration, we do not routinely intervene when this feature is seen during real-time scanning. BI-RADS 2: Benign finding. (From Cardeñosa G. Breast Imaging [The Core Curriculum Series]. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.)
FIG. 4.27 • Cyst (“complicated”). A: Oval mass with circumscribed margins, thin-edge shadows, and posterior acoustic enhancement. Internal echoes make distinction from a solid mass difficult. However, when masses with these types of internal echoes (you can almost identify the individual echoes) are seen in the subareolar area, they are commonly cysts. B: Needle (arrow) in cyst. During the aspiration, the echoes are seen being sucked into the needle along with the fluid. No residual abnormality is seen post aspiration, and thin walls are seen in these patients if a pneumocystography is done following the aspiration. On visual inspection of the fluid, the fluid in these types of cysts appears no different from that in cysts with no internal echoes. Unless we can unequivocally call a mass a cyst, however, we do an aspiration to establish the diagnosis. (From Cardeñosa G. Breast Imaging [The Core Curriculum Series]. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.)
Benign complex cystic masses that are often predominantly solid with cystic components include complex fibroadenomas (Fig. 4.35; also see Fig. 7.62), pseudoangiomatous stromal hyperplasia, and phyllodes tumors. Fat necrosis and posttraumatic changes in the acute setting are often imaged as a hyperechoic mass with cystic components (Fig. 4.36; also see Fig. 7.41C). Malignant lesions presenting as complex cystic and solid masses include invasive ductal carcinomas associated with necrosis (Fig. 4.37), malignant phyllodes, metastatic lesions, and rarely mucinous carcinomas (see Figs. 8.30, 8.33, 8.37, and 8.49) (13).
CHARACTERIZATION OF SOLID MASSES
With a solid mass, features that suggest a benign lesion include oval or ellipsoid shape, gentle bi- or trilobulation, homogeneous hyperechogenicity, and a thin, echogenic pseudocapsule (2,15) (Fig. 4.38). In classifying a mass as likely benign, a combination of features is sought, and, more importantly, no malignant features should be identified. Indeterminate features include isoechogenicity, mild hypoechogenicity, normal or enhancement of sound transmission, and homogeneous or heterogeneous echotexture. In the absence of a malignant feature or a combination of benign features, the mass is considered indeterminate, and biopsy is recommended (15).

FIG. 4.28 • Galactocele. This 28-year-old patient is breast-feeding and presents with a “lump.” She thinks it might fluctuate in size. An oval mass with indistinct margins and a heterogeneous echotexture is imaged at the site of concern to the patient. A portion of the mass is nearly isoechoic with surrounding tissue. Another portion of the mass is hypoechoic and associated with an intense shadow. Thick milky fluid is obtained on aspiration. Galactoceles are variable in their presentation. Normal skin (S) is seen well since the standoff pad was used to evaluate the patient. Also note the appearance of the tissue: homogeneous and relatively hyperechoic, a common appearance of breast tissue during lactation. (From Cardeñosa G. Breast Imaging [The Core Curriculum Series]. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.)
Table 4.3 COMPLEX CYSTIC AND SOLID MASSES
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree