Bronchiolitis refers to a wide variety of inflammatory and fibrotic disease processes affecting the small airways. Bronchiolitis is common and may be the primary manifestation in various clinical settings (e.g., infections, connective tissue diseases (CTDs), inhalational injuries, cigarette smoking, drug reactions, and transplantation). Bronchiolitis, however, frequently occurs in association with large airway disease and in association with parenchymal and interstitial lung disease ( Table 59.1 ). In this chapter the discussion is limited to conditions that primarily affect the bronchioles. Bronchiolitis associated with large airway disease and with diffuse parenchymal and interstitial lung diseases, such as hypersensitivity pneumonitis and organizing pneumonia, is discussed elsewhere.
| Bronchiolitis Associated With Large Airways Disease | Bronchiolitis Associated With Parenchymal and Interstitial Lung Disease |
|---|---|
| Chronic bronchitis/chronic obstructive pulmonary disease Asthma Bronchiectasis | Hypersensitivity pneumonitis Respiratory bronchiolitis–associated interstitial lung disease Desquamative interstitial pneumonia Lymphoid interstitial pneumonia Organizing pneumonia |
Bronchiolitis is the most common form of small airways disease. Multiple classification systems have been proposed, some of which are based on the clinical features along with the presumed etiology and the pulmonary or systemic diseases with which it is associated, some on computed tomography (CT) findings and others on histologic features. Histologic classification into cellular (proliferative) or constrictive (fibrotic) bronchiolitis is valuable as it correlates most directly with the imaging features of small airways disease. Given the nonspecific nature of histologic findings, interpretation in the context of the clinical and radiologic findings is crucial. Therefore, in this chapter, we use a classification system based on histologic features in the clinical and radiologic contexts ( Table 59.2 ).
| Cellular Bronchiolitis | Constrictive Bronchiolitis |
|---|---|
| Infectious bronchiolitis Aspiration bronchiolitis Respiratory bronchiolitis Follicular bronchiolitis Diffuse panbronchiolitis | Postinfectious Transplantation Connective tissue diseases Inhalational lung diseases Diffuse idiopathic pulmonary neuroendocrine cell hyperplasia Miscellaneous |
Anatomic Features of the Small Airways and Secondary Pulmonary Lobule
Small airways by definition are airways having a diameter of less than 2 mm. Those include membranous bronchioles, respiratory bronchioles, and alveolar ducts. Membranous bronchioles measure between 0.5 and 1 mm in diameter and are characterized by the absence of cartilage. They are lined by ciliated columnar epithelium and nonciliated Clara cells, surrounded by a layer of smooth muscle, which diminishes distally, and a layer of adventitia. Few goblet cells and seromucinous glands may be present. Terminal bronchioles, the final generation of membranous bronchioles, mark the end of the conducting division of airflow in the lungs and give rise to respiratory bronchioles, which are the beginning of the respiratory division where gaseous exchange occurs. Respiratory bronchioles can arise from terminal bronchioles or from other respiratory bronchioles and then branch into multiple alveolar ducts, alveolar sacs, and alveoli. An acinus is formed by a terminal bronchiole—with its first-order respiratory bronchioles, their branching alveolar ducts, alveolar sacs, and alveoli—and is a functional unit of the lung in which all airways participate in gas exchange.
Bronchioles maintain their mechanical support, structure, and patency through a complex network of elastic fibers attaching bronchioles to each other and to neighboring alveoli. This network is particularly important for maintaining small airway patency during expiration. Collateral ventilation occurs via small accessory channels connecting various portions of the distal small airways. Canals of Lambert provide direct connections between some membranous bronchioles and adjacent alveoli, channels of Martin provide direct interbronchiolar connections, and pores of Kohn provide interalveolar connections.
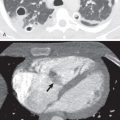
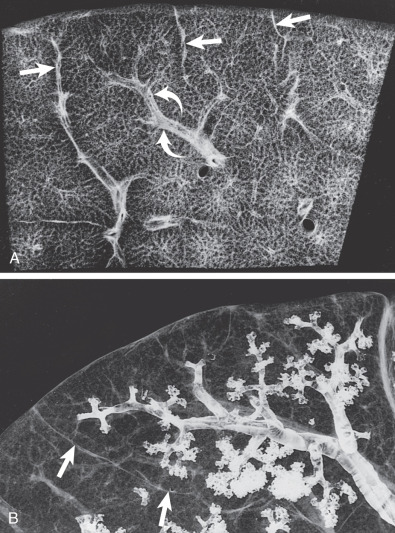
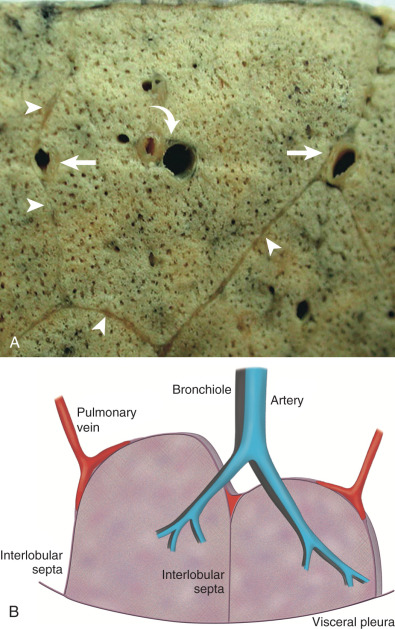
The secondary pulmonary lobule (SPL) is the smallest functioning subunit of lung. It consists of 3 to 10 acini enclosed by an interlobular septum of connective tissue and measures 1 to 2.5 cm. The bronchioles and their accompanying pulmonary artery branches are located near the center of SPLs, and the pulmonary veins are located in the interlobular septa. Thorough understanding of these anatomic features is imperative for accurate radiologic interpretation ( Figs. 59.1 and 59.2 ). Normal bronchioles cannot be identified on CT because of size limitations. The smallest intralobular structures visible on CT are intralobular pulmonary arteries measuring approximately 0.2 mm in diameter, which corresponds to the tip of the terminal bronchiole and the first-generation respiratory bronchiole ( Fig. 59.3 ). Therefore the centrilobular portion is recognized as an area around the tip of the visible pulmonary artery on CT.
Imaging of Small Airways Disease
Conventional radiography is often insensitive for detecting small airways disease, particularly in the early and localized phases of disease. Over the past several decades, technologic advances in CT have revolutionized imaging of the small airways. High-resolution volumetric datasets allow the generation of high-quality, multiplanar, and three-dimensional images. Low-dose volumetric expiratory high-resolution computed tomography (HRCT) is invaluable in detecting small airway obstruction. When used in conjunction, inspiratory and expiratory techniques provide the most reliable assessment of both the extent and severity of disease. Interpretation of characteristic radiologic findings in the appropriate clinical context is often sufficient for clinical diagnosis and initiation of treatment without biopsy confirmation, thus avoiding unnecessary invasive procedures. CT has also become a reliable, noninvasive method for assessing response to therapy without the need for repeated biopsy.
Magnetic resonance imaging (MRI) has also undergone significant progress over the past decade with efforts to overcome the limitations of other imaging modalities. Technical advances in hardware, sequencing, and contrast media have significantly improved the quality of morphologic and functional MRI assessment of airway pathology. Although MRI utility in the evaluation of small airways disease is still being researched, it has proven valuable in several other clinical settings, particularly with obstructive airway diseases, including cystic fibrosis.
Direct and Indirect Signs on Computed Tomography
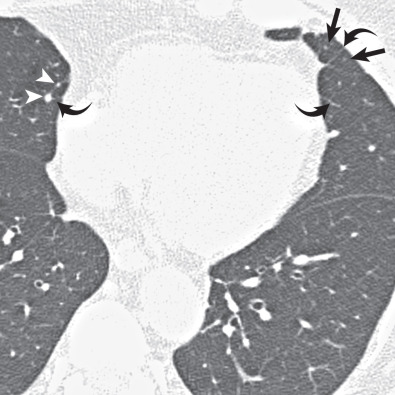
Although normal bronchioles cannot be visualized, bronchiolar diseases may result in direct and indirect signs on CT. Direct signs occur resulting from bronchiolar secretions, wall thickening, or peribronchiolar inflammation and include centrilobular nodules, branching centrilobular opacities (tree-in-bud), and occasional small centrilobular lucencies resulting from bronchiolectasis ( Fig. 59.4 ). Centrilobular opacities can be recognized because they are centered 3 mm or more from the periphery of the secondary lobule—that is, from the interlobular septa, pleura, and large pulmonary vessels. Indirect signs include areas of decreased attenuation and vascularity (mosaic attenuation) on inspiratory CT and areas of air-trapping on expiratory CT ( Fig. 59.5 ). The presence of both direct and indirect signs on CT is sensitive and specific for diagnosing small airways disease ( Table 59.3 ).
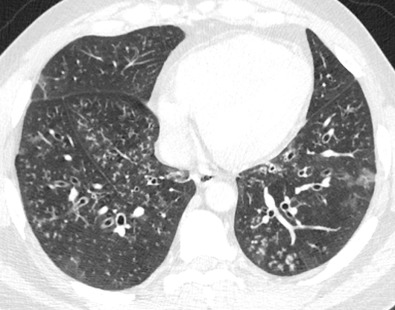
| Direct Signs | Indirect Signs |
|---|---|
| Centrilobular nodules (solid and/or ground-glass attenuation) Tree-in-bud opacities Bronchiolectasis | Mosaic attenuation (inspiratory CT) Air-trapping (expiratory CT) |
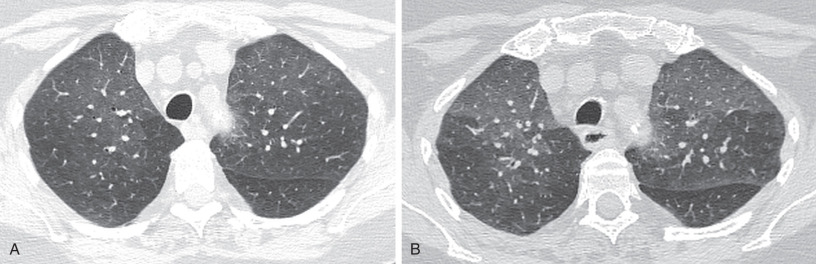
Accurate interpretation of direct and indirect signs on CT can be challenging, Indirect signs, in particular, warrant further elaboration. By definition, mosaic attenuation is an imaging pattern on inspiratory CT with differing lung attenuation as a result of variable aeration and vascular perfusion, resulting in a heterogeneous parenchymal appearance. It is associated with a broad differential diagnosis, most commonly with diseases affecting the small airways, pulmonary vasculature, alveoli, and interstitium, alone or in combination. Parenchymal lung disease is the most common cause of mosaic attenuation, seen in approximately 50% of cases, followed by small airways disease constituting nearly 33% of cases. Mild mosaic attenuation can be seen in up to 20% of normal patients. Parenchymal heterogeneity is gradient dependent, with the more dependent portions of the lung being of slightly higher attenuation. Perfusion heterogeneity, with increased perfusion and attenuation more centrally than peripherally, may also be seen.
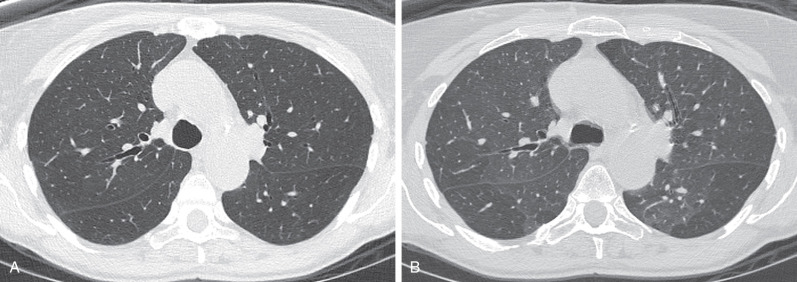
Concurrent expiratory imaging for evaluation of air-trapping is the best method for differentiating between the different causes of mosaic attenuation. In a normal expiratory CT, lung attenuation increases as the proportion of air to tissue decreases with expiration of air. The Fleischner Society defines air-trapping as “parenchymal areas with less than normal increase in attenuation and lack of volume reduction” as seen on end expiration CT scans (see Fig. 59.5B ). These areas appear as polygonal regions of low attenuation adjacent to areas of lung that have the normal increased attenuation with expiration. A simple measure of the degree of air-trapping is subdivision into mild (25%), moderate (25%–50%), and severe (50%) air-trapping, based on a subjective estimation of the total lung volume of air-trapped lung. If air-trapping is present on expiratory CT with concurrent mosaic attenuation on inspiratory CT, then small airways disease is the most likely culprit, with the hyperlucent foci representing the diseased portions of the lung ( Fig. 59.6 ). In fact, small airways disease is the most common cause of mosaic attenuation where the hyperlucent lung is the abnormal finding. As with inspiratory imaging, a similar physiologic gradient of parenchymal heterogeneity is seen on expiratory imaging. Additionally, areas of lobular air-trapping can be seen in 40% to 80% of normal patients on CT. Several studies have shown mild, moderate, and sometimes even extensive air-trapping in individuals with normal pulmonary function tests.
The specific clinicopathologic forms of bronchiolitis are discussed in the following sections.
- •
Direct signs
- •
Cause: bronchiolar secretions, bronchiolar wall thickening, peribronchiolar inflammation
- •
Manifestation: centrilobular nodules, tree-in-bud opacities, bronchiolectasis
- •
- •
Indirect signs
- •
Cause: small airway obstruction
- •
Manifestations: mosaic attenuation on inspiratory CT, air-trapping on expiratory CT
- •
Cellular Bronchiolitis
Infectious Bronchiolitis
Etiology
Acute infectious bronchiolitis is most common and severe in children, most often caused by viruses (e.g., adenovirus, respiratory syncytial virus), followed by Mycoplasma pneumonia. Less common etiologies include Chlamydia, bacteria, and fungi, particularly Aspergillus in immunocompromised patients.
Clinical Presentation
Infants and young children usually present with symptoms of an upper respiratory tract infection, followed by dyspnea, tachypnea, and fever approximately 2 to 3 days later; cyanosis and extreme weakness may be seen in severe cases. Adults are probably infected with respiratory viral infections as often as infants are; however, the severity and consequences of infection in otherwise healthy individuals are usually much less, likely because their small airways contribute less to total pulmonary resistance. Nonetheless, severe and sometimes fatal disease can occur.
Pathophysiology
Histologically, inflammatory cells (mainly neutrophils) are present within the airway walls and in inflammatory exudate and mucus in the airway lumens ( Fig. 59.7 ). These findings account for the direct signs on CT. Bronchiolar epithelial necrosis may occur in severe cases. Biopsy is rarely required for diagnosis.

Manifestations of the Disease
Radiography.
Conventional radiography in infants and children may demonstrate bronchial wall thickening (peribronchial cuffing) and peribronchial (central) areas of consolidation. Other findings include hyperinflation (caused by partial small airway obstruction) and patchy bilateral consolidations (indicating progression to bronchopneumonia). In adults findings are often subtle, manifesting as multifocal nodular or reticulonodular opacities. As with children, progression to bronchopneumonia results in patchy bilateral consolidations ( Fig. 59.8 ).

Computed Tomography/High-Resolution Computed Tomography.
Characteristic CT findings include small well-defined centrilobular nodules and tree-in-bud opacities, which can be patchy and unilateral or asymmetric and bilateral. While tree-in-bud opacities, in particular, are highly suggestive of endobronchial spread of small airways infection, they can also been seen with noninfectious etiologies (e.g., aspiration, cystic fibrosis, follicular bronchiolitis). Progression to bronchopneumonia results in 5- to 10-mm-diameter airspace nodules and patchy lobular, subsegmental, or segmental ground-glass opacities and consolidations ( Fig. 59.9 ).

Differential Diagnosis
Similar histologic and CT findings may be seen with aspiration or with CTDs, inflammatory bowel disease, and allergic bronchopulmonary aspergillosis. Acute infectious and/or aspiration bronchiolitis (AB) frequently occur in the setting of preexisting bronchiectasis.
- •
Etiology
- •
Viruses, Mycoplasma , bacteria (early bronchopneumonia), and fungi (particularly Aspergillus )
- •
- •
Symptoms
- •
Often severe in infants; usually mild in adults
- •
- •
Radiographic findings
- •
Children: peribronchial cuffing, hyperinflation
- •
Adults: bilateral nodular/reticulonodular opacities
- •
Patchy bilateral consolidations indicating bronchopneumonia
- •
- •
Computed tomography/high-resolution computed tomography
- •
Centrilobular nodules, tree-in-bud opacities, patchy ground-glass opacities, or consolidations
- •
Tree-in-bud opacities highly suggestive of small airways infection
- •
Aspiration Bronchiolitis
Etiology, Prevalence, and Epidemiology
AB refers to chronic inflammation of the bronchioles secondary to chronic and recurrent aspiration of gastric and other foreign material. The disease primarily affects individuals with oropharyngeal dysphagia and other patients at high risk for aspiration (elderly, neurologic disorders, dementia). AB may also manifest acutely in certain clinical scenarios, such as in patients presenting with drug overdose–related respiratory depression, acute trauma, or altered mental status resulting from strokes or seizures.
Clinical Presentation
Patients usually present with signs of persistent airway disease, such as increased secretions and sputum production, productive cough, wheezing, and dyspnea, all of which are exacerbated by oral intake.
Pathophysiology
Histologic findings are very similar to infectious bronchiolitis, with bronchial and bronchiolar inflammation, bronchial wall thickening, and inflammatory exudate and mucus in the airway lumens.
Manifestations of the Disease
Radiography.
Conventional radiographic findings are nonspecific and manifest as unilateral or bilateral small nodular and/or patchy opacities with or without lung hyperinflation ( Fig. 59.10 ) .

Computed Tomography/High-Resolution Computed Tomography.
As with infectious bronchiolitis, diffuse small centrilobular nodules and tree-in-bud opacities are present, which can be patchy and unilateral or asymmetric and bilateral. Patchy lobular consolidations are often present and frequently progress to confluent segmental or lobar consolidations. A distinguishing feature of aspiration is the pattern of distribution within the lungs, which is dependent on patient positioning at time of aspiration. Bedridden patients who remain in a supine position will often present with disease affecting the posterior dependent segments of the upper and lower lobes, hospitalized patients who remain in a midline semiupright position will present with lower lobe–predominant disease, and patients who spend most of their time lying on their right or left side will present with disease involving the right-dependent lung or left-dependent lung, respectively (see Fig. 59.10 ).
- •
Etiology
- •
Aspiration of gastric and/or other foreign material
- •
- •
Symptoms
- •
Persistent airway disease and productive cough exacerbated by oral intake
- •
- •
Radiographic findings
- •
Unilateral or bilateral nodular or patchy opacities, +/− hyperinflation
- •
Frequent patchy bilateral consolidations indicating bronchopneumonia
- •
- •
Computed tomography/high-resolution computed tomography
- •
Centrilobular nodules, tree-in-bud opacities, patchy ground-glass opacities or consolidations
- •
Distribution dependent on patient positioning at time of aspiration
- •
Respiratory Bronchiolitis
Etiology, Prevalence, Epidemiology, and Clinical Presentation
Respiratory bronchiolitis (RB) is predominantly a smoking-related entity, which can occur rarely in nonsmokers or with other inhalational exposures, such as asbestos, nonasbestos dusts, and various fumes. RB is present histologically in virtually all smokers and by definition is not associated with symptoms or functional impairment. When involvement is symptomatic or leads to functional decline, the process is referred to as RB-associated interstitial lung disease, discussed separately in further detail (see Chapter 34 ).
Pathophysiology
RB is characterized histologically by intraluminal and peribronchiolar accumulation of macrophages that contain a finely granular yellow-brown cytoplasmic pigment referred to as smoker’s macrophages ( Fig. 59.11 ). Other common findings include chronic peribronchiolar mononuclear cell infiltration, mild inflammation, airspace enlargement with fibrosis, and diffuse hyaline-like acellular fibrotic thickening of alveolar septa. The intensity of macrophage pigmentation and peribronchiolar fibrosis correlates with the number of pack-years smoked. Emphysema is almost always present.

Manifestations of the Disease
Radiography.
RB rarely results in any demonstrable abnormality on chest radiography. Subtle upper lung zone–predominant lucencies (representing emphysema), peribronchial cuffing, and small poorly defined nodular or patchy opacities may be present in some patients.
Computed Tomography/High-Resolution Computed Tomography.
CT is usually normal or may show mild centrilobular and/or paraseptal emphysema. When present, findings include small, multifocal, poorly defined ground-glass centrilobular nodules; patchy ground-glass opacities; and mosaic attenuation ( Fig. 59.12 ). Findings most commonly are upper lung zone predominant, however, and may be diffuse in some patients. Several studies have shown that smoking cessation does not always lead to complete resolution of the radiologic and pathologic features of RB, with the extent of disease often remaining similar among current smokers and ex-smokers, even years after smoking cessation.

Differential Diagnosis
The differential diagnosis for RB on CT includes hypersensitivity pneumonitis (HP), follicular bronchiolitis (FB), infectious bronchiolitis, cholesterol granulomas, pulmonary capillary hemangiomatosis, and occasionally pneumoconioses. Significant radiologic overlap may be seen with cases of RB and the subacute inflammatory phase of HP, which includes centrilobular ground-glass nodules, patchy ground-glass opacities, and mosaic attenuation on inspiratory CT. Although CT manifestations may be diffuse in both conditions, RB tends to produce a more upper lung zone–predominant pattern when compared with HP. Conversely, focal/lobular air-trapping is more commonly seen with HP on expiratory CT. Thus an upper lung zone–predominant or diffuse imaging pattern with concurrent findings of emphysema favors the diagnosis of RB, although diffuse parenchymal involvement with areas of lobular air-trapping favors subacute HP. However, as RB and HP are clinically and histologically distinct entities, a multidisciplinary approach integrating clinical history, histology, and imaging findings is imperative for accurate diagnosis. Recent studies have shown that the combination of smoking history, typical CT findings, and lack of lymphocytosis on bronchoalveolar lavage is highly suggestive of RB. Furthermore, cigarette smoking has been shown to have a protective effect against the development of HP. For example, in a study of 400 consecutive patients with various interstitial lung diseases, only 6% of 116 patients with a final diagnosis of hypersensitivity pneumonitis were smokers, compared with 20% of the 284 remaining patients. Cholesterol granulomas and pulmonary capillary hemangiomatosis are two entities seen in the setting of pulmonary hypertension that may also manifest with centrilobular ground-glass nodules. Of importance, these entities should be considered when centrilobular ground-glass nodules are seen in conjunction with known pulmonary hypertension or an enlarged pulmonary trunk on CT.
- •
Etiology/definition
- •
Accumulation of pigmented macrophages in the lumen of respiratory bronchioles and adjacent alveoli
- •
Almost always associated with smoking
- •
- •
Symptoms
- •
By definition, not associated with symptoms or functional impairment
- •
- •
Radiographic findings: usually normal
- •
Computed tomography/high-resolution tomography
- •
Often normal or showing only emphysema
- •
Poorly defined centrilobular nodules, patchy bilateral ground-glass opacities, predominantly in the upper lobes or diffuse
- •
Pulmonary Lymphoid Hyperplasia (Follicular Bronchiolitis)
Etiology
Pulmonary lymphoid hyperplasia or FB is characterized by peribronchial and peribronchiolar inflammation secondary to the abundance of lymphoid follicles. Several clinical conditions are associated with FB, which are identical to those seen with lymphoid interstitial pneumonia, most commonly in association with CTDs (particularly rheumatoid arthritis [RA] and Sjögren syndrome), immunologic disorders (Hashimoto thyroiditis, pernicious anemia, autoimmune hemolytic anemia, chronic active hepatitis, primary biliary cirrhosis, and myasthenia gravis), congenital or acquired immunodeficiency diseases (common variable immunodeficiency, human immunodeficiency virus/acquired immune deficiency syndrome), systemic hypersensitivity reactions, infection, allergy (including asthma), bone marrow transplantation, and as a reactive process distal to bronchiectasis or in association with middle lobe syndrome.
Clinical Presentation
FB occurs most frequently among middle-aged adults, presenting with progressive dyspnea and cough. Less common symptoms include fever, weight loss, and recurrent pneumonia. Pulmonary function tests may reveal an obstructive, restrictive, or mixed pattern. FB may occasionally be seen in children.
Pathophysiology
FB is characterized histologically by the presence of abundant lymphoid follicles in bronchiolar walls and, to some extent, along bronchi, interlobular septa, and pleura. Obstructive pneumonia may sometimes occur as a result of airway compression.
Manifestations of the Disease
Radiography.
FB findings on radiography tend to be subtle, and in many cases the chest radiograph may be normal. When findings are present, they usually manifest as bilateral reticular or reticulonodular opacities ( Fig. 59.13 ).

Computed Tomography/High-Resolution Computed Tomography.
Typical FB results in small diffuse and poorly defined centrilobular nodules. Other less common and variable findings include peribronchial and subpleural nodules, patchy ground-glass opacities, bronchial wall thickening, and mosaic attenuation. Expiratory CT may show lobular areas of air-trapping. In one study CT showed bilateral centrilobular nodules (100%), patchy ground-glass opacities (75%), peribronchial nodules (42%), and subpleural nodules (25%) (see Fig. 59.13 ).
- •
Etiology
- •
Peribronchial and peribronchiolar inflammation resulting from abundance of lymphoid follicles
- •
Associated with multiple clinical conditions: CTDs, most commonly rheumatoid arthritis, immunologic disorders, immunodeficiency, systemic hypersensitivity, infection, hematopoietic stem cell transplantation
- •
- •
Symptoms
- •
Progressive dyspnea and cough
- •
Less commonly, fever, weight loss, recurrent pneumonia
- •
- •
Radiographic findings
- •
Often normal
- •
Bilateral nodular or reticulonodular opacities
- •
- •
Computed tomography/high-resolution tomography
- •
Poorly defined small bilateral centrilobular nodules
- •
Patchy bilateral ground-glass opacities
- •
Peribronchial nodules/bronchial wall thickening
- •
Subpleural nodules
- •
Mosaic attenuation on inspiratory CT; lobular air-trapping on expiratory CT
- •
Diffuse Panbronchiolitis
Etiology, Prevalence, and Epidemiology
Diffuse panbronchiolitis is a progressive form of cellular bronchiolitis associated with chronic inflammation of the respiratory bronchioles and paranasal sinuses. Etiology and pathogenesis remain unknown; however, the disease has been recognized almost exclusively in Asia, particularly in Japan. This distribution is believed to be highly associated with genetic predisposition located between human leukocyte antigens A and B loci. The average age of onset is approximately 40 years; male-to-female ratio is approximately 2 : 1.
Clinical Presentation
Diffuse panbronchiolitis manifests insidiously with productive cough and progressive dyspnea, worse on exertion. Features of chronic sinusitis are also typical. Pseudomonas aeruginosa frequently colonizes the respiratory tract of affected individuals. Pulmonary function tests reveal a marked obstructive and mild restrictive pattern.
Pathophysiology
Histologically, mononuclear inflammatory cells (predominantly lymphocytes, plasma cells, and foamy histiocytes) are found within the walls of respiratory bronchioles, alveolar ducts, and, to a lesser extent, adjacent alveoli. Mucus and aggregates of neutrophils may be seen within the airway lumen. The late stage of the disease is frequently complicated by Pseudomonas aeruginosa colonization , which is associated with a significantly worse prognosis. In one study the 10-year survival rate for those infected with the organism was only 12%, compared with 73% for those who remained uninfected.
Manifestations of the Disease
Radiography.
Chest radiography may show small diffuse nodular or reticulonodular opacities, peribronchial cuffing, and mild to moderate hyperinflation.
Computed Tomography/High-Resolution Computed Tomography.
CT often shows diffuse small centrilobular nodules, tree-in-bud opacities, mosaic attenuation, bronchiolectasis, and bronchiectasis. The presence of these findings correlates to the stage of the disease; the earliest manifestation consists of centrilobular nodules, followed by tree-in-bud opacities, bronchiolectasis, and eventually bronchiectasis. Cystic bronchiectasis may be seen in the late stage ( Fig. 59.14 ).