Abstract
Bronchopulmonary sequestration is a congenital developmental lung pathology characterized by a supernumerary lung tissue that receives its blood supply by a systemic feeding artery usually originating from the descending aorta. The diagnosis may be establish by a routine fetal ultrasound during the second or third trimester through identification of a homogeneous, hyperechoic, solid lung mass with an aberrant feeding systemic artery. It generally has a good prognosis and a high rate of spontaneous resolution, but the prognosis depends mainly on the mass size and the presence of fetal fluid effusions. In the presence of hydrothorax or hydrops, perinatal mortality is almost 100%. For such high-risk cases, fetal surgery by full laser ablation of the feeding artery has been demonstrated to be the best procedure to improve prognosis and to avoid the need of postnatal intervention. At the same time, cases that have a good prognosis with expectant management during pregnancy require a postnatal surgical resection of the lung mass.
Keywords
bronchopulmonary sequestration, hyperechogenic lung mass, fetal surgery
Introduction
Bronchopulmonary sequestration (BPS) is a solid lung lesion of nonfunctioning pulmonary tissue, a supernumerary lobe of the lung, which lacks connection to the tracheobronchial tree and receives its blood supply from an aberrant systemic feeding artery, originating commonly from the descending aorta. Two forms are recognized: intralobar sequestration (ILS), which is incorporated into normal lung tissue, and extralobar sequestration (ELS), which has its own pleural cover and is completely separated from the normal lung. ELS may also be outside the thoracic cavity.
Disorder
Prevalence and Epidemiology
Bronchopulmonary sequestration is the second most common congenital lung anomaly after congenital cystic adenomatoid malformation (CCAM). ELS accounts for most cases of BPS diagnosed prenatally and 25% to 50% of cases of BPS diagnosed postnatally. This difference in prenatal and postnatal prevalence is thought to be explained by the fact that many instances of ILS could be acquired postnatally. ELS was reported to have 4 : 1 male predominance, but recent series report a similar prevalence in both sexes.
Etiology and Pathophysiology
Bronchopulmonary sequestration frequently occurs in the lower left lung. It is thought to originate from a supernumerary caudally positioned lung bud that migrated caudally during lung development together with the esophagus. Development before or after formation of the pleura leads to ILS or ELS, respectively. ELS can be intrathoracic (usually on the left, comprising up to 50% of all cases); located in the anterior and posterior mediastinum (8% and 6%); or outside the chest cavity, most frequently below the diaphragm (up to 18% of cases). ELS and ILS forms of BPS can coexist. ELS has a systemic arterial blood supply from supradiaphragmatic or infradiaphragmatic vessels, normally originating directly from the aorta. ELS masses usually, but not always, have venous connection to systemic vessels (superior vena cava, azygous vein, and hemiazygous vein), while ILS always drains to pulmonary veins.
Manifestations of Disease
Clinical Presentation
Extralobar sequestration is usually diagnosed prenatally as an intrathoracic or abdominal mass, with characteristic ultrasound (US) and Doppler features. The prenatal prognosis depends on the mass size and the presence of hydrothorax or hydrops. BPS generally has a good prognosis and a high rate of spontaneous resolution. Of BPS diagnosed prenatally, 75% of masses dramatically regress on serial prenatal US scanning, being detectable only by postnatal cross-sectional imaging studies. The mechanism of regression could relate to spontaneous thrombosis of the systemic feeding artery.
A small proportion of cases show large masses that can cause massive hydrothorax and severe mediastinal shift with fetal hydrops. A possible additional mechanism for fetal hydrops is arteriovenous shunting through the lung mass and high-output cardiac failure. Compression of the esophagus can also cause polyhydramnios and preterm delivery in some cases.
Neonatal outcome depends on several factors, as follows:
- •
Mass size and position. Large intrathoracic masses may lead to lung hypoplasia, mediastinal shift, and fetal hydrops owing to blood vessel compression. Intraabdominal ELS generally has a good prognosis.
- •
Development of massive pleural effusion and/or fetal hydrops. Those cases are usually at risk of intrauterine fetal demise.
- •
Associated anomalies.
The risk of chromosomal abnormalities has not been found to be significantly increased in isolated BPS.
Imaging Technique and Findings
Ultrasound.
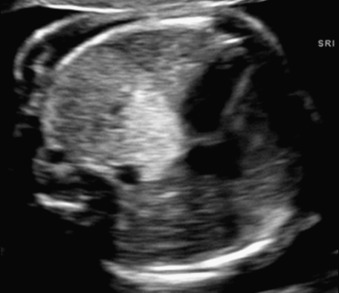
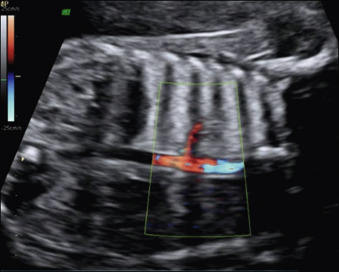
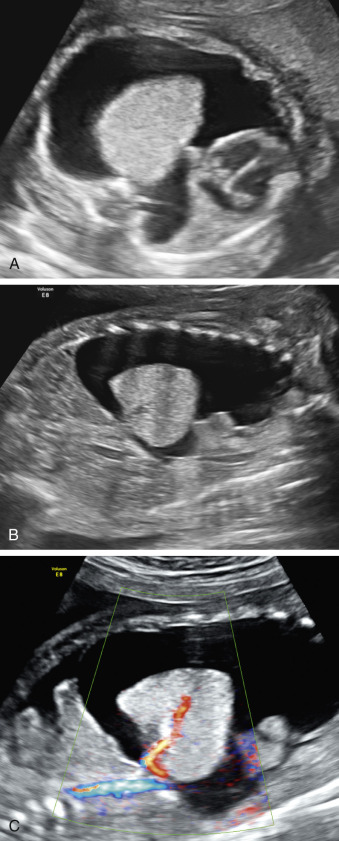
Bronchopulmonary sequestration typically appears as a hyperechogenic, mostly solid, homogeneous, well-defined lesion ( Fig. 3.1 ). Usually detected in the second trimester, it commonly shows slight growth at the beginning and subsequent, sometimes dramatic, regression later during the pregnancy. If ELS is situated below the diaphragm, it may be indistinguishable from a suprarenal mass. The pathognomonic sign of BPS is the presence of a systemic feeding artery that can be identified by using color or power Doppler US as arising from the descending aorta and entering into the lung mass ( Fig. 3.2 ).
Hydrothorax may occur in rare cases of ELS. If large in size, the lung mass and hydrothorax can cause mediastinal shift and hydrops ( Fig. 3.3 ). The mechanism for hydrothorax has been speculated to include also increased venous return because of shunting through the affected lung.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree