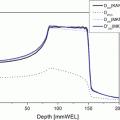
Fig. 15.1
DVH analysis was performed to compare optic nerves that did and did not develop toxicity
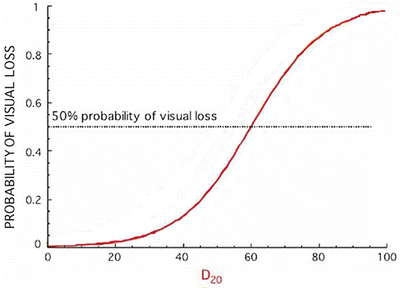
Fig. 15.2
Probability of visual loss as a function of D 20 was calculated according to an integral logistic model
Every effort should be made to spare the uninvolved optic nerve so that bilateral visual acuity loss can be avoided. Dose constraints at NIRS have been previously published, in which the most relevant parameter is D 20. Preservation of visual acuity at a risk of <5 % can be achieved if D 20 is maintained lower than 30 GyE. It is important to evaluate the risk on the basis of the whole nerve dose-volume histogram (DVH) and not only the specific dose or volume.
15.3.3.1 Optic Nerve Sparing
The GTV (Fig. 15.3, dark orange line) is contoured according to contrast MRI, CT, and MET-PET images, and image fusion is routinely employed. A shrinking-field technique is employed in head and neck cancer. The larger CTV1 (Fig. 15.3, light orange line) receives the first 9 fractions and the smaller CTV2 receives the last 7 fractions. Addition of uniform geometric margins around the GTV would result in CTV1 and CTV2, which were drawn similarly for avoidance of irradiation to the right optic nerves (Fig. 15.3, blue contours). Irradiation of the left optic nerve was unavoidable (Fig. 15.4).
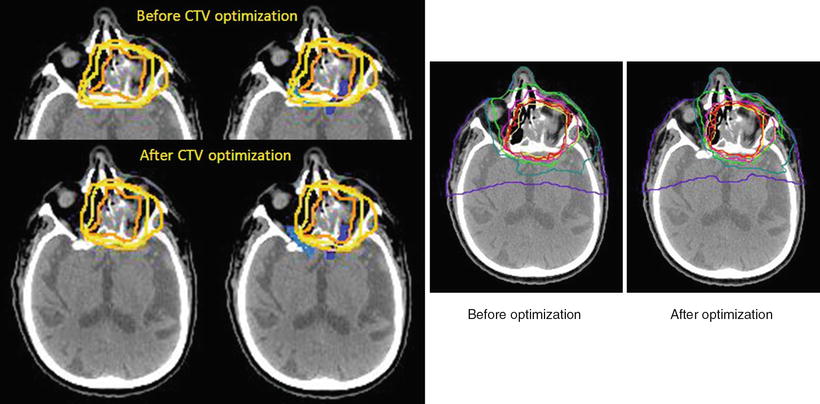
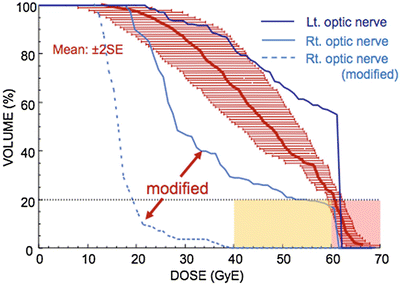

Fig. 15.3
Before CTV optimization, both optic nerves were included in the high-dose area. After CTV optimization, good sparing of the right optic nerve was achieved. Isodose level: red = 96 %; orange = 90 %; magenta = 70 %; green = 50 %; cyan = 30 %; purple = 10 %. Contour: dark orange = gross tumor volume, light orange = large clinical target volume, yellow = small clinical target volume

Fig. 15.4
Probability of visual loss: red zone 58 %, yellow 30 %, pink 50 %. Relatively small changes in CTV can produce a dramatic effect on the optic nerve DVH
This 76-year-old male patient with ACC was treated in December 2008. The prescribed dose was 64 GyE in 16 fractions over 4 weeks. At 37 months after C-ion RT, the patient is alive, with local control and preservation of visual acuity in both eyes.
15.3.4 Osteonecrosis
In radiation therapy, blood supply to the bone may be inhibited at the microscopic level. In particular, if the bone is destroyed by the tumor and irradiated with high-dose carbon ions or photons, the bone may undergo necrotic changes and subsequent infection may occur. Osteonecrosis caused by both tumor involvement and irradiation is extremely difficult to treat and can cause severe pain. Surgical resection of necrotic bone (sequestrectomy) may be necessary for pain control. The jawbone is also more prone to radiation-induced necrosis because of exposure to the oral bacterial load.
When the bone infiltrated by the tumor is irradiated at a high dose (for instance, in sarcoma cases), the risk of bone necrosis may be unavoidable. Oral care with weekly irrigation has the potential of lowering this risk. When bone necrosis develops, it is important to administer conservative treatment first, with systemic antibiotics, analgesics, and local antiseptic agents. Surgical resection may be necessary if the patient experiences severe pain. Close cooperation between the radiation oncologist and the oral and maxillofacial surgeon is required, and treatment planning should be optimized to allow future implant surgery (for instance, sparing zeugmatic processes whenever possible).
15.4 Results of Therapy
Between April 1997 and August 2012, a total of 438 cases were treated using 57.6 or 64.0 GyE. Histologically, 175 patients had ACC, 102 had MMM, 50 had adenocarcinoma, and the others had different diagnoses. With regard to the tumor site studied, the paranasal sinus was studied in 119 cases, the nasal cavity in 81, the major salivary gland in 59, the oral cavity in 54, the pharynx in 51, and other sites in the remaining cases. Almost all patients (74 %) had inoperable tumors.
The 5-year local control rates according to major histological types in our institution were as follows: 81 % for adenocarcinomas, 74 % for ACCs, and 79 % for MMMs. With regard to the prescribed tumor dose, the 5-year local control rates of ACC were 81 % for the 64 GyE group and 69 % for the 57.6 GyE group. Although this rate did not differ significantly between these groups (p = 0.0789), the local control rate for the 64 GyE group tended to be better. However, 14 patients with bone and soft tissue sarcomas in the head and neck region (mostly osteosarcomas) had a poor 5-year local control rate, although a majority of the patients received 64 GyE. Consequently, a new treatment protocol was introduced in April 2001 to apply a total irradiation dose of 70.4 GyE in 16 fractionations over 4 weeks (see Chap. 17), a schedule similar to that used for the treatment of bone and soft tissue sarcomas of the trunk.
Additionally, the 5-year overall survival rates were 57 % for adenocarcinomas, 72 % for ACCs, and 33 % for MMMs. Although the local control facilitated by C-ion RT was promising for MMM, the survival rate was not commensurate with the favorable local control rate because of subsequent regional lymph node or distant metastases. Further to the results of preliminary analysis of this study, a new protocol was introduced in April 2001 for the purpose of prophylactic therapy against distant metastasis, the major cause of death in MMM of the head and neck region (see Chap. 16).
Regarding late radiation morbidities, almost all of the late skin and mucosal reactions were of grade 1 or lower. Less than 5 % of the patients developed grade 2 skin or mucosal reactions. The recorded tumor-related events that needed surgical treatment included encephalitis, sinusitis, otitis media, and maxillary bone necrosis. These events were documented in patients that had been informed before the start of therapy about the possibility of adverse events due to tumor infiltration. Other patients reported no unexpected serious adverse reactions.
15.5 Case Studies
15.5.1 Skin Reaction
15.5.1.1 A Case with Severe Skin Reaction
A 59-year-old man with MMM was treated with C-ion RT in July 1997. A bulky tumor invading the right nasal and paranasal cavity was observed on enhanced MRI (Fig. 15.5). The prescribed dose was 57.6 GyE in 16 fractions over 4 weeks. During the early phase of the dose escalation study, most of the tumors were treated using only two portals from the antero-posterior and lateral directions, with the safety margin being drawn equally around the GTV or CTV. Therefore, when the tumor invaded or was close to the overlying skin, it was also irradiated with the same dose as was administered to the tumor. This treatment was applied in the current case, in which the cheek skin was included for irradiation with high-dose levels, as shown in the Fig. 15.5b.
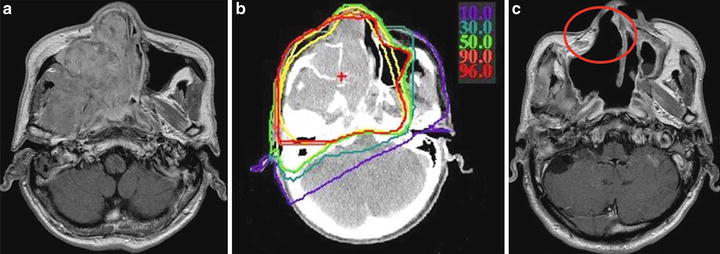

Fig. 15.5
Gadolinium-enhanced T1WI images and isodose distribution. (a) Before treatment, (b) isodose distribution, (c) after treatment. Isodose level: red = 96 %; orange = 90 %; green = 50 %; cyan = 30 %; purple = 10 %. Contour: yellow = clinical target volume
At 52 months after C-ion RT, the patient showed complete disappearance of the tumor but developed external fistula of the skin (Figs. 15.5c and 15.6-middle) corresponding to the high-dose area (Fig. 15.6-left).
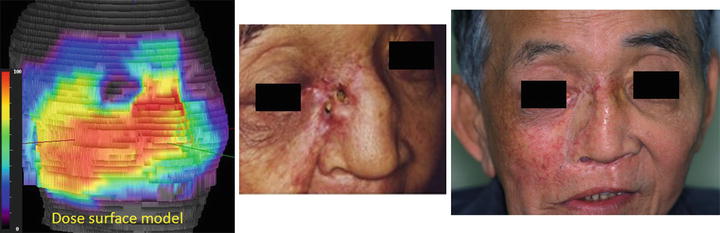

Fig. 15.6
Dose-surface model and skin reaction. The image on the right side is taken after skin grafting
In this case, only two portals were used and no specific plan for sparing the skin was considered at the time. A wide surface of the skin received the full dose. Local control was achieved, but a severe late effect developed corresponding to the high-dose area. Although this patient had received surgery for skin grafting (Fig. 15.6-right), he survived 58 months after C-ion RT and died of intercurrent diseases.
15.5.1.2 A Case with Acceptable Skin Reaction
A 63-year-old woman with MMM of the left nasal and paranasal cavity was treated with C-ion RT from January to February 2007. The prescribed dose was 64.0 GyE in 16 fractions over 4 weeks. In this case, a very tight margin toward the cheek skin was set to reduce the dose to the skin (Fig. 15.7).
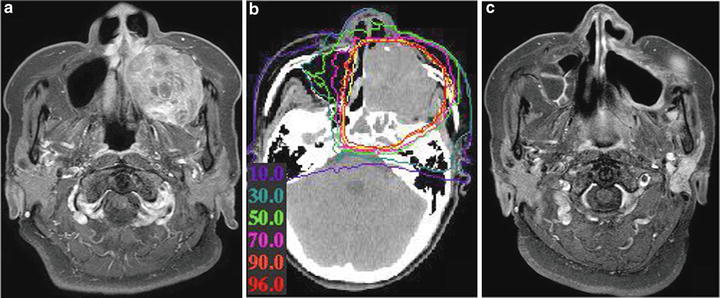

Fig. 15.7
Gadolinium-enhanced T1WI images and isodose distribution. (a) Before treatment, (b) isodose distribution, (c) after treatment
Twenty-five months after C-ion RT, the patient is alive without tumor progression. No significant late toxicity is present apart from a right facial nerve deficit, which was already present at diagnosis (Fig. 15.8).
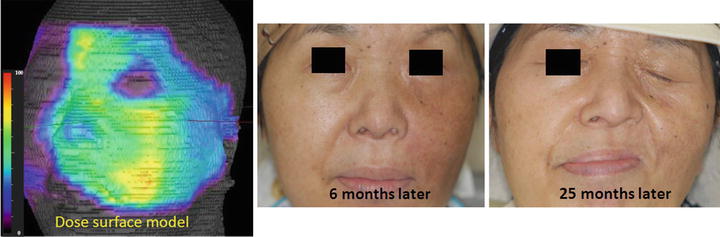





Fig. 15.8
Dose-surface model and skin reaction
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree