Key Points
- ▪
The initial evaluation of cardiac masses should be performed using echocardiography.
- ▪
Cardiac CT scanning is able to image cardiac masses and can contribute to their evaluation. The superior field of view of CCT compared with both transthoracic echocardiography and TEE may assist with the assessment of paracardiac masses.
- ▪
From the pathology point of view, it is important to recall that imaging and histology are distinct entities.
- ▪
The goal of CCT should be to determine whether a mass demonstrates malignant features such as tissue plane violation.
Although CCT is able to image most lesions within the heart, especially larger ones, its role is secondary to those of echocardiography and MRI in the evaluation of lesions within the heart. CCT’s greatest contribution is to evaluate the thorax in detail for complex cardiac masses (both those extending into the heart and those located elsewhere). Neither transthoracic nor transesophageal echocardiography has sufficient field of view to see beyond the heart into the chest cavity or even entirely around the heart. For example, a pericardial cyst often is not apparent by echocardiography (unless the cyst abuts the chest wall).
Lesions that are atypical for myxomas should be further evaluated by CT scanning to ensure that there are no signs of malignant disease elsewhere within the chest or abdomen.
CT scanning is unparalleled in its ability to identify calcification within lesions, such as pericardial masses or thickening.
Although CCT is able to image small and mobile structures within the heart, such as vegetations, echocardiography, by virtue of having vastly superior temporal resolution, is the preferred test for imaging small and mobile lesions within the heart itself. For any mass lesion other than the most obvious myxoma, it would be unwise not to perform a CT scan of the chest and abdomen to exclude signs of malignancy elsewhere.
CCT may assist with surgical planning in cases such as the younger patient with presumed atrial myxoma by confirming lesional characteristics as seen by echocardiography and by excluding significant coronary artery disease.
CCT can image a wide range of intra- and paracardiac structures:
- □
Intracardiac masses and lesions
- •
Vegetations
- •
Thrombi
- •
Tumors/masses
- •
Abscesses
- •
- □
Paracardiac masses and lesions
- •
Cysts
- •
Tumors/masses
- •
Cardiac Masses
Cardiac masses can be categorized with respect to nature:
- □
Thrombus
- □
Cysts
- □
Abscesses
- □
Neoplasms
Thrombus
Thrombus, the most common type of intracardiac mass, is most often located within the left atrial appendage. It is often associated with mitral valve disease and underlying atrial dysrhythmias.
In the left ventricle, thrombi usually are apical in location and usually are associated with prior myocardial infarct. This may occur in an area of associated severe hypokinesis, or within a left ventricular aneurysm. Left ventricular thrombi can be laminar, more rounded and mass-like, or pedunculated. Long-standing thrombi can calcify. Pre–contrast-enhanced and delayed imaging can be used to aid in differentiating between a non-enhancing thrombus and an enhancing tumor.
If a thrombus is suspected within the right atrium, in the absence of an instigating factor such as a central line or pacing wire, one must attempt to exclude tumor or tumor thrombus from an intra-abdominal source such as renal cell carcinoma or hepatocellular carcinoma.
Cysts
Pericardial Cysts
For discussion and examples of pericardial cysts, see Chapter 17 .
Echinococcosis
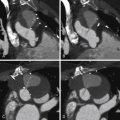
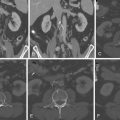
Echinococcosis of the heart, also known as hydatid disease or echinococccal disease, is the result of parasitic infection with the tapeworm Echinococcus granulosus . The parasite is shared in its life cycle with another mammal such as a dog or sheep, and the disease is endemic in sheep-ranching areas. The incubation period is months to years. Echinococcus eggs lead to the growth of cysts of 5 to10 cm in size that characterize the disease and may involve the heart ( Fig. 23-1 ).

Abscesses
For a discussion and examples of cardiac abscesses, see Chapter 20 .
Cardiac Neoplasms
Cardiac neoplasms are rare. They include, in order of frequency:
- 1.
Metastases (the most common, by 20- to 40-fold)
- 2.
Malignant primary neoplasms
- 3.
Benign primary neoplasms
In a consecutive autopsy series of 12,485 cases, the prevalence of primary cardiac tumors was 0.056% and of cardiac metastases 1.23%.
Metastases
Metastatic cardiac disease spreads to the heart by four pathways:
- □
Retrograde lymphatic extension: lung, breast, lymphoma
- □
Hematogenous spread: melanoma, breast, lymphoma
- □
Direct contiguous extension: lung, esophagus, breast, lymphoma, thymoma
- □
Transvenous extension: kidney, liver (up the inferior vena cava), bronchogenic (down a pulmonary vein)
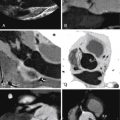
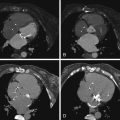
The most common primary sources for cardiac metastases include lung bronchogenic carcinoma, breast adenocarcinoma, lymphoma, melanoma, and sarcomas ( Figs. 23-2 through 23-6 ).






Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree