Chapter 3 Cardiac Magnetic Resonance Imaging
INTRODUCTION
Cardiac magnetic resonance imaging (CMRI) provides reproducible morphologic and functional information for the evaluation and management of patients with cardiovascular disease. CMRI has improved substantially over the past decade, and it is now entering the mainstream of diagnostic cardiac imaging. CMRI is already considered the procedure of choice for quantification of ventricular volume and mass, as well as in the evaluation of myocardial viability, pericardial disease, and intracardiac and pericardiac masses, for imaging the right ventricle and pulmonary vessels, and for assessing many forms of congenital heart disease, especially after corrective surgery.
MAGNETIC RESONANCE IMAGING TECHNIQUES
Spin Echo Magnetic Resonance Imaging
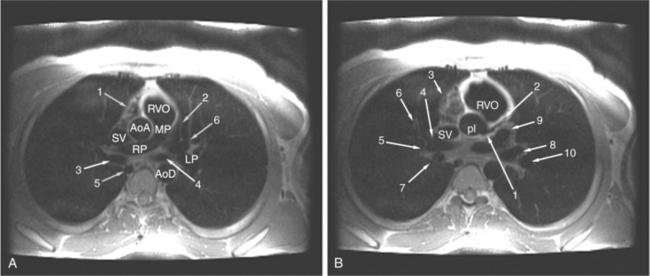
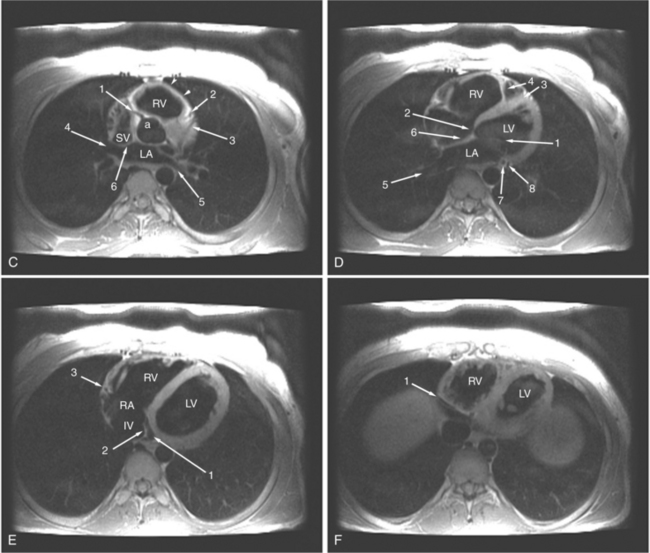
ECG gated spin echo (SE) MRI provides imaging with the highest contrast resolution, resulting in high anatomic detail (Fig. 3-1). Furthermore, characterization of wall thickness and content of the arterial wall provides tissue-specific findings encouraging early diagnosis. Adaptation of k-space segmentation to the acquisition of image data allows rapid image acquisition within single breath holds. These techniques (namely, turbo SE and double inversion recovery) are actually modified gradient echo pulse sequences and provide the same high contrast imagery of conventional SE in dramatically less imaging time.
Multi-Echo Spin Echo Imaging
In an SE acquisition, a rephasing pulse is applied at a time interval after the spins have dispersed, resulting in an SE. If these spins are allowed to diphase again, their signal decreases, and if a second rephrasing pulse is applied, a second SE is obtained, of signal exponentially less than the first. If this process is continued, using a chain of rephrasing pulses, a series of sequentially exponentially decreased signal SEs are obtained. The loss of signal over time is directly related to the transverse relaxation time (T2) of a particular tissue. Thus, imagery obtained with each successive SE will display a map of signal intensities reflecting the T2s of the tissues in the imaging field (Fig. 3-2). The more or less rapid loss of signal in a particular region or tissue can then be used to characterize that tissue (i.e., tissues that lose signal rapidly over the chain of echoes have shorter T2 than those that lose signal less rapidly). This technique may be useful for differentiating cystic from solid masses or for enhancing the appearance of interstitial tissue involvement in a disease process.
Gradient Echo Magnetic Resonance Imaging
Gradient echo (GE) cine imaging allows a short acquisition time. The bright signal of the blood pool in these images results from flow-related enhancement obtained by applying intermittent rapid radiofrequency pulses to saturate a volume of tissue. The images may be reconstructed in the different phases of the cardiac cycle and can be displayed in cine format (Fig. 3-3). Consistent imaging artifacts caused by flow accelerating across a luminal stenosis or turbulence within a dilated chamber may be used to identify these conditions and to assess their significance (Fig. 3-4). Cine loop display demonstrates dynamic changes in the morphology of the heart, providing a means for evaluating regional wall motion, ventricular function, and valvular dysfunction. GE images may be analyzed quantitatively, providing accurate indices of ventricular function and valvular dysfunction.
Phase Contrast (Blood Flow) Mapping
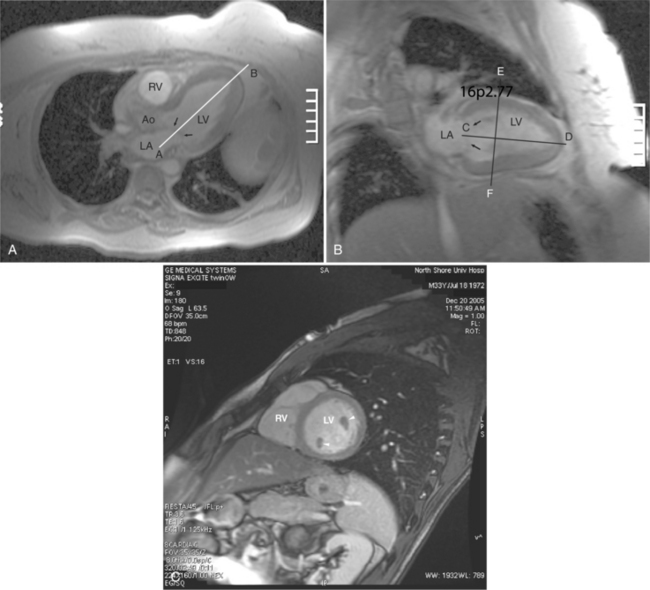
When a pulse sequence is applied to a patient in an MRI scanner, two sets of data are obtained. The “real” (amplitude) data contains the map of protons within the slice, that is, the “stuff” of a cross-sectional image. The other set of data obtained is the “imaginary” (phase) data. This latter data provides a map of the net velocity of the protons within the slice. The intensity of a pixel in an imaginary image reflects the velocity and phase of the protons within that pixel. Following the intensity of a region of pixels in a series of images provides us with time-intensity curves reflecting the flow of blood through a portion of the heart in a manner analogous to the peak velocity measured by continuous wave Doppler echocardiography. This technique uses modified GE sequences with image reconstruction from the phase rather than the amplitude of the MR signal. On phase images, the gray value of a pixel depends on velocity and direction with respect to the imaging plane (Fig. 3-5). Thus, the flow mapping technique allows determination of the peak velocity of blood flow within a stenosed blood vessel or total flow through a vessel over time. Flow velocity, flow volume, and mean blood flow may be quantitated within areas of interest.
Myocardial Tagging
By applying saturation planes perpendicular to the imaging plane at the electrocardiographic trigger signal before image acquisition, tagging sequences create noninvasive markers within the heart wall. During image acquisition, reduced signal is obtained from the presaturated tissue, resulting in images formed with orthogonal or radial black lines on the images (depending on the method of tagging). Because the tag lines are a property of the tissue (i.e., the slice of the heart), the lines move with the myocardium through the cardiac cycle. When created at end diastole, the lines deform as the myocardium contracts and then become undeformed as the myocardium relaxes. Tracking the motion of the tag lines through the cardiac cycle allows visual evaluation of intramural myocardial deformation (Fig. 3-6). In this way intramyocardial motion can be evaluated. Application of sharp, closely spaced tag lines allows qualitative or quantitative analysis of myocardial deformation from which strain analysis, that is, change in the shape of the myocardium, can be performed.
Perfusion and Delayed Hyperenhancement Imaging
Rapid imaging over the heart after bolus intravenous contrast administration will produce a series of images displaying the passage of the contrast through the cardiac circulation. Contrast enhancement of the blood pool within the right atrium and ventricle is followed, after passage through the lungs, to the cavities of the left atrium and left ventricle, and then via the coronary circulation into the interstitial space of the ventricular myocardium (Fig. 3-7). Areas of decreased myocardial blood flow appear as relative signal voids within a segmental distribution corresponding to the upstream arterial narrowing. Thus, regional distribution of blood flow and the assessment of myocardial perfusion can be performed in much the same manner as in a nuclear perfusion examination. The sensitivity of detecting myocardial ischemia is improved by imaging after administration of a pharmacologic vasodilator. A hemodynamically significant coronary arterial stenosis is present if coronary blood flow cannot be increased by vasodilator stimulus. When hybrid echo planar pulse sequences and pharmacologic vasodilatation are used, 87% to 90% sensitivity and 85% specificity for detecting significant coronary stenosis can be obtained. Gadolinium-chelate contrast material remains in the extracellular space when administered by intravenous route. If the normal signal of ventricular myocardium is nulled by administration of a presaturation pulse, delayed imaging 10 minutes after intravenous contrast administration visualizes the heart after the contrast has cleared the cardiac cavities and the myocardium, revealing a relative signal void in normal myocardium (Fig. 3-8). Delayed washout kinetics and an increased volume of gadolinium distribution in the interstitial space of abnormal myocardium result in delayed myocardial enhancement. This technique has been found useful in characterizing myocardial infarction and fibrosis in other cardiac disorders.
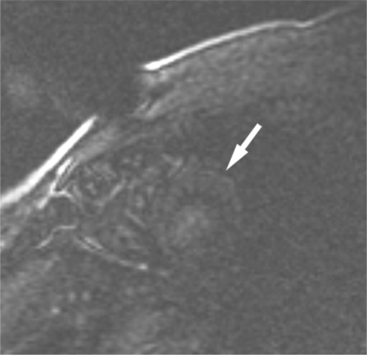
FIGURE 3-8 Short-axis image of the heart obtained 10 minutes after intravenous administration of Gd-DTPA (see Figure 3-7A) and immediately after a presaturation pulse. The viable left ventricular myocardium (arrow) is barely visible.
In this chapter, we will review the use of ECG-gated MRI techniques for the diagnosis and evaluation of patients with acquired heart disease. This chapter details the process of planning, performing, and interpreting a CMRI examination. We focus our comments on discussing pathophysiologic mechanisms and recognizing their effect on cardiac morphology and function as displayed in MR images.
PERICARDIAL DISEASES
Normal Pericardium
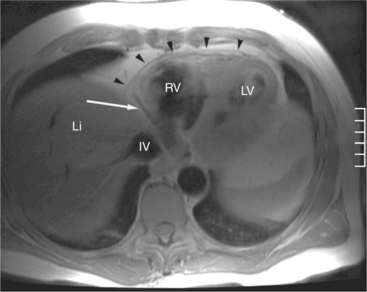
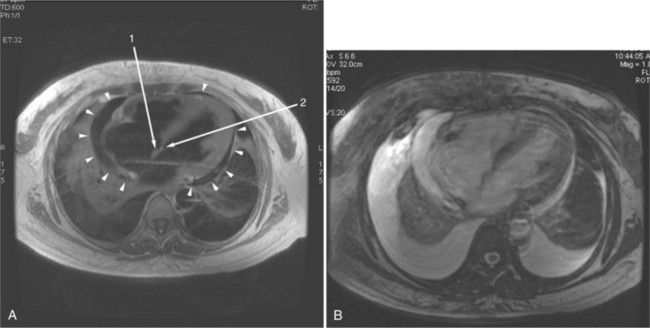
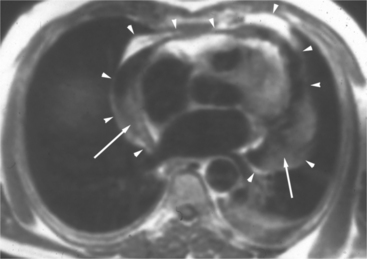
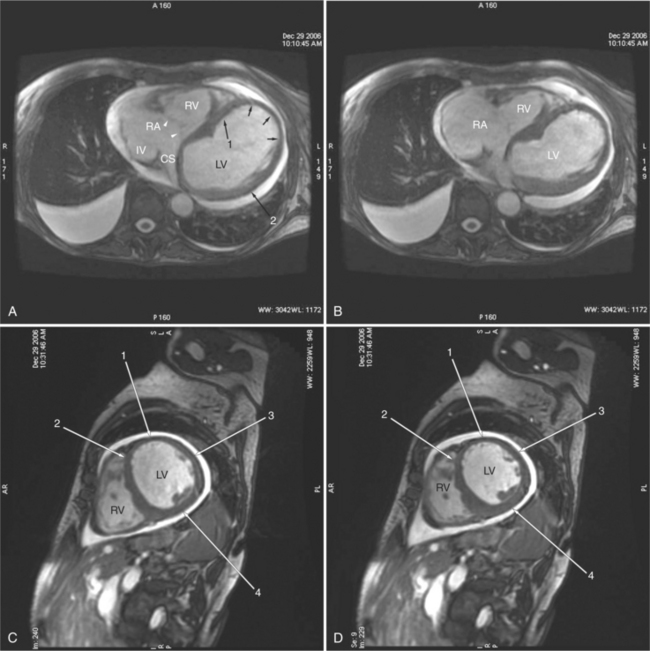
The visceral pericardium is normally thin and not visualized separately by any imaging modality. The combination of the visceral pericardium and the small volume of physiologic pericardial fluid constitutes the normal pericardium routinely visualized on MRI as a 1- to 2-mm-thick layer, which can appear focally thicker at the sites of its major attachments. On SE MR the normal pericardium appears as a pencil-thin line of low signal intensity between the epicardial and pericardial fat (Fig. 3-9). The low signal is attributed to the fibrous nature of the parietal pericardium, the low protein content of pericardial fluid, and the nonlaminar flow patterns caused by cardiac pulsation.
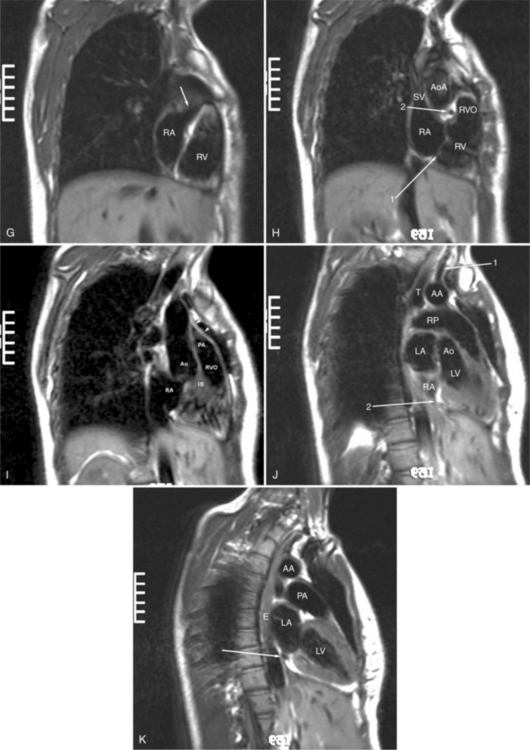
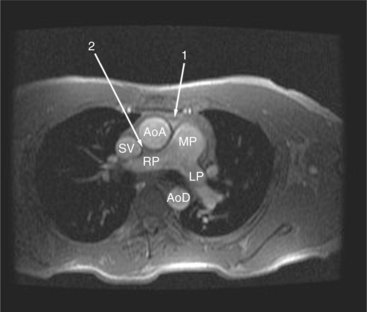
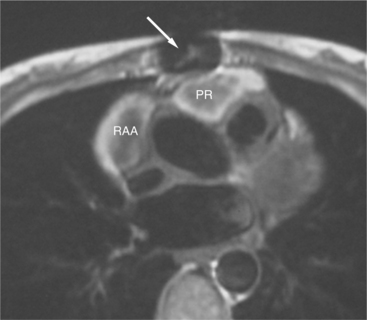
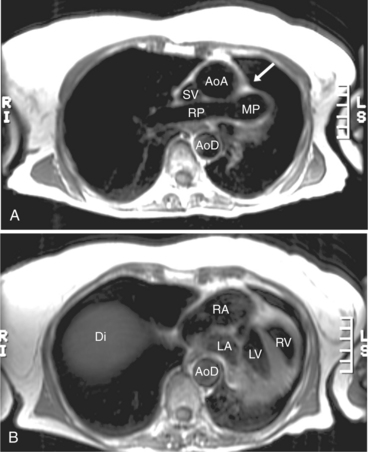
The reflection of pericardium around the great arteries and veins forms the two pericardial “appendages.” Anterior to the aorta this contiguous pericardial space is called the “preaortic recess,” whereas posteriorly it is called the “retroaortic” or superior pericardial recess (Fig. 3-10). Posterior and lateral to the heart, the extraparenchymal pulmonary veins and the superior and inferior venae cavae are enveloped by the pericardium. The intrapericardial space between the pulmonary veins is called the oblique sinus. It is essential to appreciate the anatomic extent and location of these pericardial sinuses since they are normally seen on MR.
Pericarditis and Pericardial Effusions
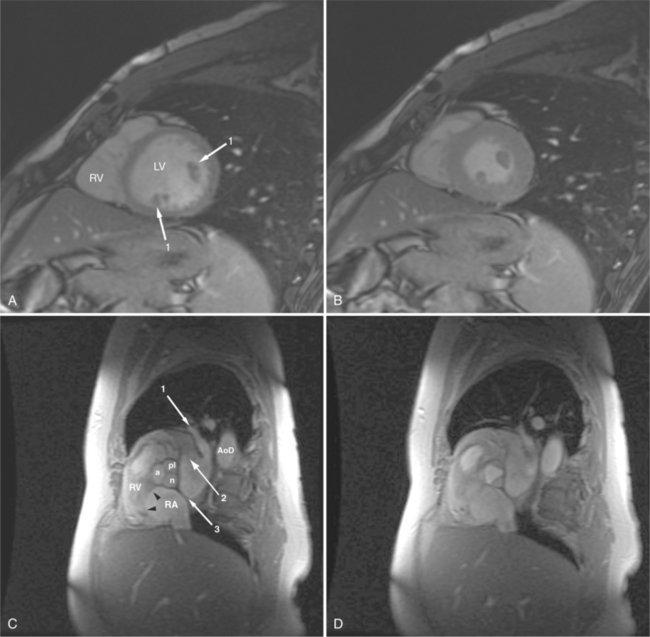
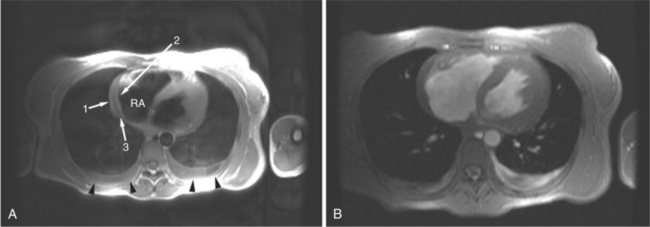
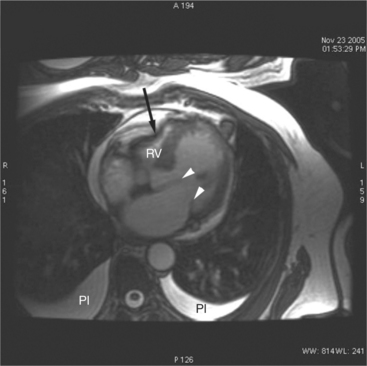
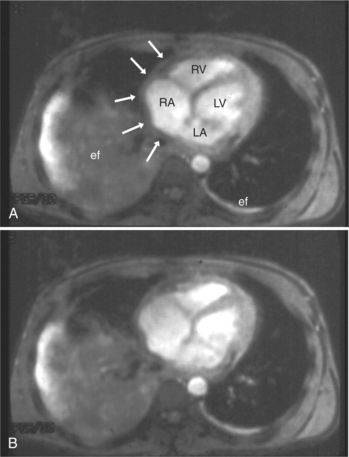
Normal pericardium most commonly responds to insult by cellular proliferation or the production of fluid. Pericarditis results in pericardial thickening. The abnormal pericardium is characterized by intermediate signal intensity on both SE and GE examination (Fig. 3-11). The most common manifestation of acute pericarditis is an effusion. The character of the fluid varies with the underlying cause of the effusion. Transudative pericardial effusion may develop after cardiac surgery or in congestive heart failure, uremia, postpericardiectomy syndrome, myxedema, and collagen-vascular diseases (Fig. 3-12). Hemopericardium may be found after trauma, aortic dissection, aortic rupture, or in cases of pericardial neoplasm (especially primary pericardial mesothelioma). The typical appearance of common pericardial effusion in increased distance between the epicardial and pericardial fat, characterized by low-to-absent signal on SE and bright signal on GE images. Thus, pericarditis (with or without associated effusion) and pericardial effusion are differentiated by the signal of the pericardium and its contents.
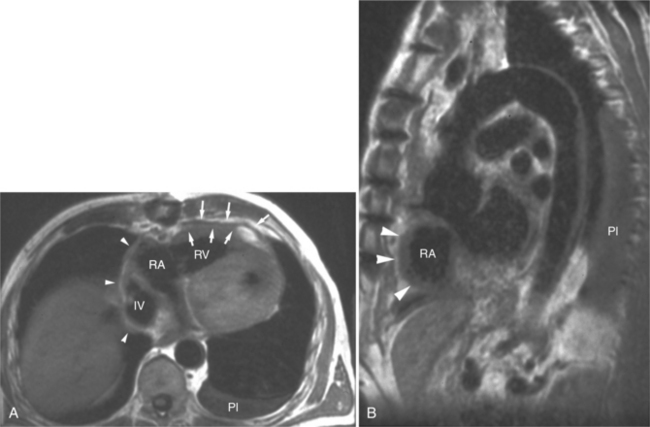
On SE examination, hemorrhagic pericardial effusion presents as areas of mixed low, intermediate, and high signal, depending on the age of the blood. Nonhemorrhagic effusions on SE MR have predominantly low signal intensity as a result of spin phase change of the pericardial fluid. GE MR sequences display freely mobile pericardial fluid as high signal intensity. The high protein content of inflammatory pericardial fluid seen in uremia, tuberculosis, or trauma may have intermediate signal intensity components on SE MR, especially in dependent areas (Fig. 3-13). Furthermore, because adhesions are common in pericardial inflammation, inflammatory effusions may not have the normal free flow patterns of pericardial fluid leading to loci of increased signal intensity on SE sequences similar in appearance to loculated pericardial effusions. Pericardial inflammation, as seen in uremic or tuberculous pericarditis or trauma following resuscitation, appears as increased signal intensity as compared with myocardium on SE MR acquisition (Fig. 3-14).
Congenital Absence of the Pericardium
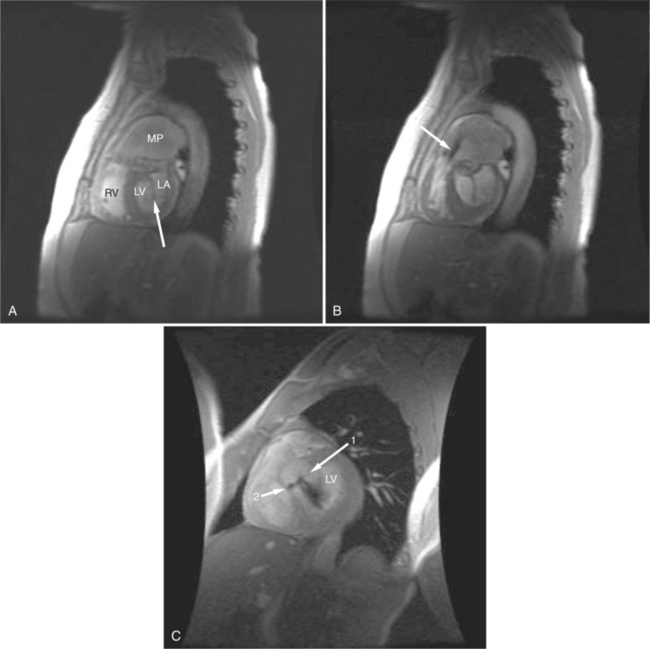
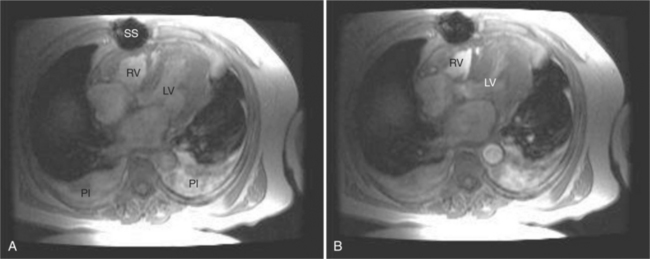
Absence of the pericardium is thought to be a result of compromise of the vascular supply to the pleuropericardial membrane that surrounds the ventral cardiac tube during embryologic development. Pericardial defects may vary in size from small communications between the pleural and pericardial cavities to complete (bilateral) absence of the pericardium. The most common form is complete absence of the left pericardium, with preservation of the pericardium on the right side (Fig. 3-15).
Pericardial Cysts and Diverticula
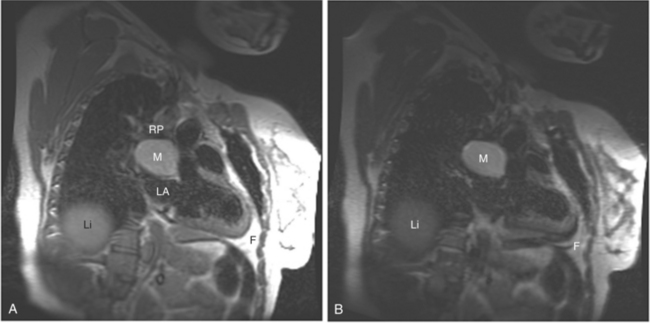
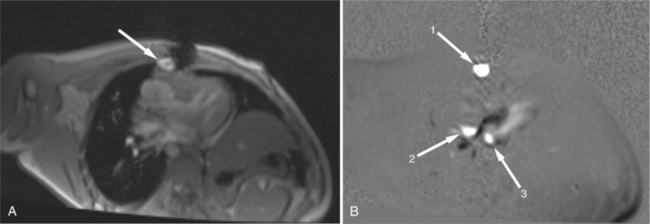
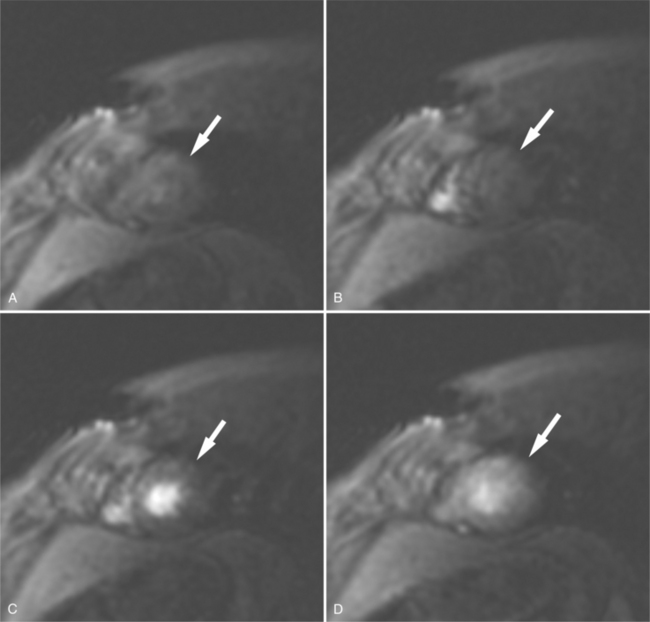
If a portion of the pericardium pinches off completely from the pleuropericardial membrane during embryologic development, a pericardial cyst forms, containing the same mesothelial lining as the normal pericardium (Fig. 3-16). Similarly, a pericardial diverticulum is a cyst that fails to completely separate, leaving persistent communication with the pericardial space. SE MR acquisition depicts these findings as fluid-filled paracardiac masses. If multi-echo acquisition is obtained, then the cyst appears to increase in signal (with respect to the surrounding organs) on longer echo time (TE) images (see Figure 3-2).
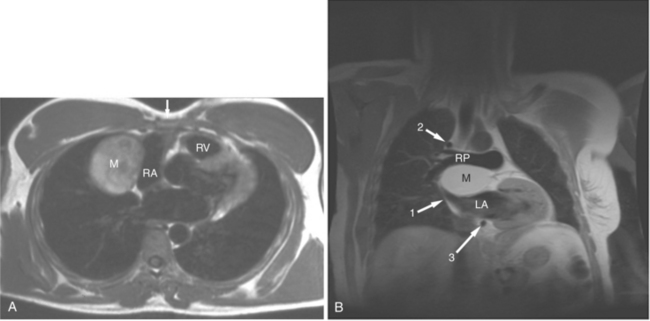
FIGURE 3-16 Two patients with pericardial cysts. A, Axial double inversion acquisition from an asymptomatic 26-year-old man with an unusual chest film. A large, circumscribed, nearly homogeneous high signal intensity mass (M) extrinsically compresses the right atrium (RA) and displaces the heart toward the left. Notice how the right ventricular (RV) free wall lies to the left of the sternum (arrow). B, Coronal spin echo acquisition from a 40-year-old woman with an unusual chest film (same patient as in Figure 3-2). A circumscribed, homogeneous, high signal intensity mass (M) elevates the right pulmonary artery (RP) and compresses the left atrium (LA) and right upper lobe pulmonary vein (arrow 1). This is an intrapericardial pericardial cyst. The azygos vein (arrow 2) and coronary sinus (arrow 3) are viewed in cross section.
Pericardial Tamponade
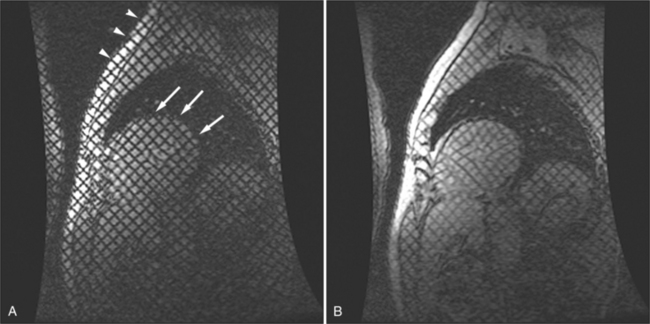
The rapid accumulation of as little as 100 to 200 ml of fluid can impede diastolic ventricular filling and lead to pericardial tamponade. Pericardial tamponade occurs when reduced stroke volume limits maintenance of cardiac output. Although MRI is not the initial diagnostic modality for evaluating tamponade, it is frequently instrumental in suggesting the cause of the effusion (i.e., hemorrhage, neoplastic involvement, inflammation resulting from tuberculosis or other infectious processes) in this acutely emergent situation. Double inversion recovery demonstrates pericardial thickening and a “tubular” right ventricle (Fig. 3-17). Cine MRI may be useful to demonstrate diastolic atrial or ventricular collapse (Fig. 3-18). In many cases, the motion of the interventricular septum is paradoxical. Earlier right ventricular filling results in diastolic bowing of the septum toward the left ventricle.
Pericardial Constriction
The hallmark of pericardial constriction is pericardial thickening (with or without pericardial calcification) and abnormal diastolic ventricular function. In the majority of cases, constrictive pericarditis involves the entire pericardium, compromising filling of both the right and left heart. Occasionally, however, local chronic pericardial thickening has been reported. Focal pericardial thickening is more commonly seen in the postoperative patient and is frequently located anterior to the right ventricle (Fig. 3-19). The clinical findings of constrictive pericarditis overlap with those of restrictive cardiomyopathy, a primary disorder of the myocardium. Differentiation between these two entities is imperative because patients with pericardial constriction may benefit from pericardiectomy; myocardial restriction may be rapidly progressive and necessitate cardiac transplantation. In pericardial constriction, the right ventricle may appear tubular in appearance. Gradient echo acquisition demonstrates decreased right ventricular contractile function and limited diastolic excursion, common to both restriction and constriction (Fig. 3-20). Dilatation of the right atrium, venae cavae, coronary sinus, and hepatic veins, reflecting right heart failure may be found in cases of constrictive pericarditis and in cases of restrictive cardiomyopathy. Abnormal right heart filling in constriction is visualized as early diastolic right atrial or right ventricular collapse and the septal “bounce,” which is early reversal of septal curvature.
Symptomatic pericardial constriction may be found in the absence of conventional radiographically detectable pericardial thickening, however. Pericardial thickening is not diagnostic of pericardial constriction; demonstration of pericardial thickening greater than 4 mm in face of characteristic hemodynamic findings distinguishes constrictive pericarditis from restrictive cardiomyopathy.
Myocardial Ischemia and Infarction
Cardiac MR has become the clinical gold standard for quantitation of cardiac chamber volume and myocardial mass. The accurate and reproducible quantitative data obtained from MR examination makes GE MRI appealing as an imaging modality for long-term follow-up of patients with ischemic heart disease. MRI is valuable for assessment of regional and global contractile function; clinically, this is usually achieved by visual inspection of cines in standard imaging planes. Quantification of wall motion and thickening using conventional techniques is possible for both the left ventricle and the right ventricle. Regions of myocardial ischemia or infarction appear as areas of thinned ventricular myocardium and segments of decreased or absent contraction (Fig. 3-21).
Quantitation of ventricular function is based on GE acquisition and planimetry of the endocardial and epicardial contours of images obtained at intervals in the cardiac cycle (Fig. 3-22). The volume of the left ventricular chamber within each slice is the planimetered endocardial area multiplied by the slice thickness (Fig. 3-23). The left ventricular end-diastolic volume is calculated as the sum of the volumes of the slices of the heart through the left ventricle obtained at end diastole. Similarly, the left ventricular end-systolic volume is the sum of the slice volumes obtained at end systole. Ventricular stroke volume is the difference between end-diastolic volume and end-systolic volume. Ejection fraction is the stroke volume indexed to (divided by) the end-diastolic volume. Cardiac output is the stroke volume multiplied by the heart rate.