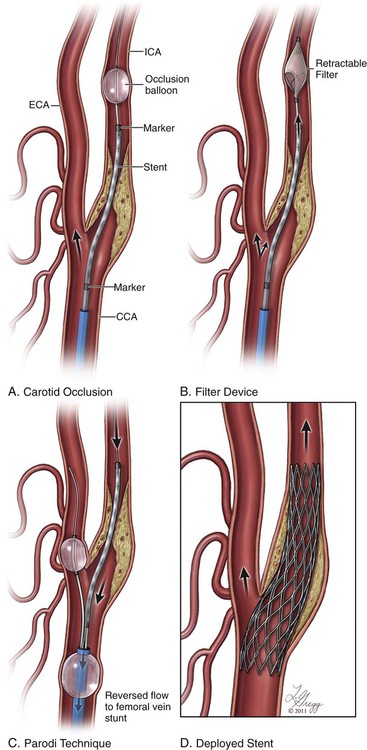
Stroke represents the third leading cause of death in the United States, with an incidence of 1.5 deaths per thousand people. Of the more than half-million strokes occurring annually, occlusive disease of the extracranial circulation is responsible for approximately 30%. The traditional standard of care in treating cervical carotid artery occlusive disease has been carotid endarterectomy (CEA), a procedure initially performed in the 1950s and described by Scott, DeBakey, and Cooley. In 1988, the landmark North American Symptomatic Carotid Endarterectomy Trial (NASCET) demonstrated a reduction in stroke and death rates from 26% at 2 years to 9% after endarterectomy.1 Since that time, additional studies have further validated this approach and demonstrated the benefit of intervening in patients who are asymptomatic from their disease.2–4 Currently there are no absolute contraindications for carotid artery stenting (CAS). Relative contraindications to the use of CAS can be divided into anatomic and patient-specific factors. Anatomic factors are physician and trial specific, but general contraindications are similar to those outlined in the CREST trial5 (asymptomatic lesions < 70% by ultrasound or < 60% by angiography and symptomatic lesions < 70% by ultrasound or < 50% by angiography). Lesion characteristics are also increasingly recognized as being pertinent to the risk of stroke. Lengthy lesions, those with appearance of extensive thrombus (either by conventional angiography or by intravascular ultrasonography), those with globular calcifications, and those with type III and type IV arches are at increased risk and must be approached with caution. Inability to pass the protective filter, and a tortuous vessel in which the angiographer believes he or she is unlikely to pass the stent must be warning flags and must cause the performing physician to have a low threshold for abandoning the procedure. The aortic arch is the “Achilles heel” for carotid stenting, and if the angiographer has difficulty accessing the vessel of interest, with extensive manipulation of the aorta and multiple catheter exchanges, it is best to abandon the procedure and consider alternative methods of treatment. • Intolerance/resistance to aspirin and/or clopidogrel • Patient with active bleeding diathesis or absolute contraindication to anticoagulation Aspirin and clopidogrel are the most common dual platelet therapy in use today. Resistance to antiplatelet agents is not uncommon and can result in in-stent restenosis or thrombosis. Resistance can be assessed by platelet aggregometry. Regarding clopidogrel, some studies have shown that greater than 50%6 of patients undergoing cerebrovascular stent placement might be low responders, and 0% to 44%7 may be resistors, which can be related to genetic polymorphisms of cytochrome P450 3A4 and the P2Y12 receptor. Alternatives are available for low responders/resistors and include increasing the clopidogrel dose, ticlopidine, prasugrel, or ticagrelor. Resistance to aspirin is far less common, occurring in approximately 5%6 of patients undergoing cerebrovascular stent placement. Endovascular stents come in two designs: balloon-expandable and self-expanding. Balloon-expandable stents, while appropriate for ostial lesions at the aortic arch, should not be used for treatment of cervical carotid arterial lesions, owing to their lack of flexibility or ability to spontaneously reexpand after external compression. Self-expanding stents have essentially two designs: open cell and closed cell. Each design has certain advantages and disadvantages. The open-cell design has more flexibility, which allows for better vessel wall apposition as well as better deliverability through tortuous anatomy. However, open-cell designs have lower radial force, which may result in a weaker scaffold and thus compromise stent expansion due to recoil, especially in heavily calcified lesions. Closed-cell stent designs have more radial force, but they are less flexible and can introduce kinks and stenosis in the blood vessel when used in tortuous anatomy. Data from the Stent-Protected Angioplasty versus Carotid Endarterectomy (SPACE) trial suggest that there is a higher rate of embolic complications with open-cell designs (11%) than with closed-cell carotid stents (5.6%, P = 0.029).8 Several studies have been performed to assess the outcome of incomplete stent apposition; many of these involve intracranial9 and coronary stenting.10,11 With respect to carotid stenting, the Wallstent can at times have incomplete apposition at the edges of the stent when deployed at vascular turns.12 Existence of a segment of incomplete stent apposition has no adverse morphologic or clinical effect.13 • Rx Acculink (Abbott Laboratories, Abbott Park, Ill.) • NexStent (Boston Scientific) • ADAPT (Stryker Corp., Kalamazoo, Mich.) There are two types of EPDs: (1) those that provide protection distal to the treatment site by blocking flow or filtering flow in the ICA distal to the treatment site, and (2) flow-reversal devices, which occlude inflow to the common carotid artery and external carotid artery causing reversal of flow in the ICA (Fig. 91-1). Devices that provide occlusion distal to the treatment site may consist of a balloon or, more commonly, a filter attached to a wire. The balloon occlusion concept originally pioneered by Theron has been refined.14 A balloon is attached to the working wire and advanced distal to the stenosis and inflated (PercuSurge [Medtronic, Minneapolis, Minn.]). After stenting and angioplasty are completed, a catheter is then advanced and used to aspirate debris prior to deflating and recovering the balloon. Disadvantages of the balloon system include occluding the entire flow during all or a majority of the procedure in patients who frequently have a compromised contralateral carotid artery and circle of Willis circulation. In addition, the angiographer cannot evaluate the lesion fluoroscopically while the balloon is inflated in the ICA. There is also a risk of flushing embolic debris retrograde into the common carotid and aorta or into collateral vessels off the external carotid artery, such as the middle meningeal and orbital branches. • Boston Scientific Filter Wire EZ (Boston Scientific) • Mednova-NeuroShield (Abbott) • SpideRX (ev3/Covidien, Minneapolis, Minn.) An operator should become familiar with one or two systems. Currently there are two flow-reversal devices being tested; they are based on the Parodi design.15
Carotid Revascularization
Contraindications
Equipment
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Radiology Key
Fastest Radiology Insight Engine