Central Nervous System
Helen A. Shih
Paul H. Chapman
Marc R. Bussière
Clark C. Chen
Jay S. Loeffler
Patients with a diverse mix of malignant and benign tumors within the central nervous system (CNS) can often benefit from radiation therapy. Protons improve dose conformality, thereby potentially reducing adverse treatment effects. Higher linear energy transfer (LET) heavier charged particles with a higher relative biological effectiveness (RBE) may have a possible therapeutic advantage for hypoxic and selected other tumors (see Chapter 2). In some situations, particle therapy can also be given in higher doses than photons without increasing the normal tissue injury probability. Whereas this is generally true for radiotherapy across all body sites, CNS irradiation can carry the serious consequences of cranial neuropathies or neurocognitive dysfunction. Deficits are generally delayed in onset and may arise months to years following completion of treatment. Particularly for patients with a variety of benign intracranial lesions and for some indolent malignant brain tumors, late neurologic toxicities can markedly affect long-term quality of life; the therapeutic benefit for charged particles, therefore, might be particularly prominent for these tumors.
IMMOBILIZATION AND TREATMENT PLANNING
Immobilization Techniques for Central Nervous System Fractionated Proton Radiation Therapy
A variety of external frames have been developed for patient immobilization during standard fractionated proton radiation treatment using doses ≤3 Gy (RBE). Proton beams are more sensitive than x-rays to the shape and density variations of immobilization equipment. This must be accounted for during both design of immobilization and treatment planning. In addition to the great efforts by physicians and staff to achieve accuracy and reproducibility of the immobilization, patient compliance is imperative. Although rarely required in the adult population, sedation or anesthesia must be used when this is a problem.
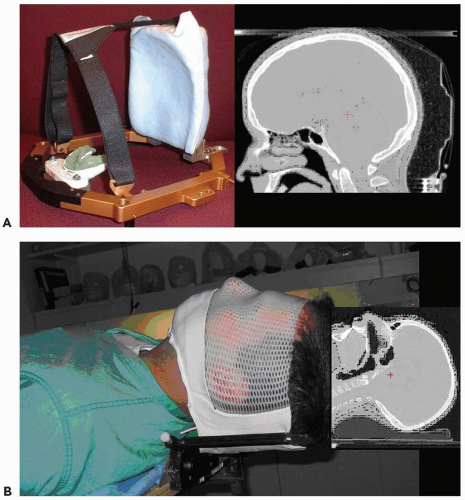
At the Massachusetts General Hospital (MGH), two immobilization devices have been developed for proton therapy of brain tumors. The MGH-modified Gill-Thomas-Cosman (GTC; Integra-Radionics Inc. Burlingtion, MA) frame (MGTC) incorporates a rounded carbon fiber occipital support and low-density cushion in addition to the GTC frame (see Fig. 12.1A). This device is used to treat intracranial targets that do not extend to the base of skull. This device provides excellent reproducibility for patients with adequate dentition. Custom dental molds are an integral part of the immobilization frame.
An alternate device is the intracranial (IC) mask, which utilizes a standard head-cup and a custom-designed polyfoam mask of perforated thermoplastic reinforced with a similar but solid sheet of polyfoam (Fig. 12.1B). The base of the IC device is made of carbon fiber to permit treatment beams to be employed from any angle. This device allows treatment fields to extend lower than the MGTC frame and can be used with patients with poor or no dentition.
Immobilization Techniques for Central Nervous System Proton Stereotactic Radiosurgery
Intracranial proton stereotactic radiosurgery (PSRS), as with other modalities for radiosurgery, relies on accurate patient
immobilization and alignment. At MGH, a multiday process has been adopted. Consented patients undergo a minimally invasive procedure for placement of three radiopaque markers within the outer table of the skull. Markers can be chosen by institutional preference but 1-mm diameter stainless steel spheres have been used for many years at MGH without complication. With the use of local anesthetic to three areas of the scalp (typically bilateral frontal and a single posterior parietal sites), a 3-mm pit is drilled percutaneously into the outer table of the skull and the stainless steel fiducial inserted. The procedure requires approximately 15 minutes with virtually no blood loss and excellent patient satisfaction. The fiducial markers define the reference coordinates for alignment which are obtained from subsequent computed tomography (CT) imaging.
immobilization and alignment. At MGH, a multiday process has been adopted. Consented patients undergo a minimally invasive procedure for placement of three radiopaque markers within the outer table of the skull. Markers can be chosen by institutional preference but 1-mm diameter stainless steel spheres have been used for many years at MGH without complication. With the use of local anesthetic to three areas of the scalp (typically bilateral frontal and a single posterior parietal sites), a 3-mm pit is drilled percutaneously into the outer table of the skull and the stainless steel fiducial inserted. The procedure requires approximately 15 minutes with virtually no blood loss and excellent patient satisfaction. The fiducial markers define the reference coordinates for alignment which are obtained from subsequent computed tomography (CT) imaging.
Treatment Planning and Delivery for Central Nervous System Proton Radiation Therapy
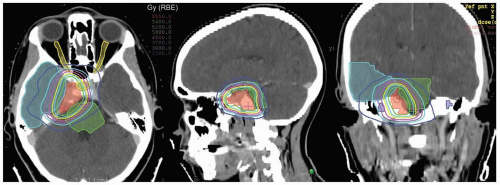
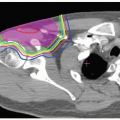
Since proton ranges are determined by converting CT densities into stopping power, contrast material has the effect of overestimating the required range when beams are aimed through vascular areas of the brain. This is overcome by using precontrast scans for range and dose calculations that are then fused with contrast enhanced scans for target and critical structure delineation. Targets and critical structures are outlined. Between one and six optimized beams are used, depending upon the target. Careful port design should maximize sharp lateral and distal dose falloff by avoiding beams directed tangential to bony ridges or through heterogeneous areas such as the mastoids, sinuses, and auditory canals. Field-specific collimation and distal range compensation are used to provide conformal beam shaping. Pristine peaks with various ranges are superimposed to create a flat spread-out Bragg peak (SOBP). The dose distribution is typically far more uniform regardless of target dimensions compared to photon-based treatments for either fractionated or single-fraction therapies. Frequently excellent sparing of surrounding normal structures from irradiation can be achieved (see Fig. 12.2).
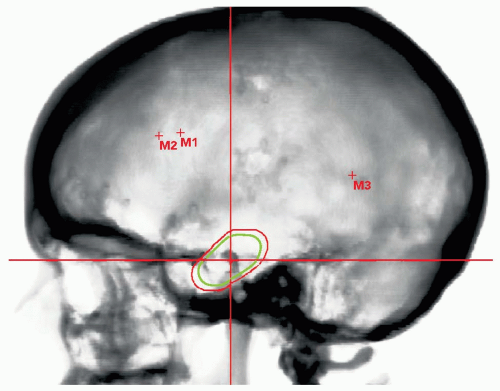
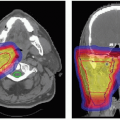
Daily high precision treatment alignment is achieved with onboard diagnostic imaging. Full three-dimensional (3-D) analysis using implanted fiducial markers is used for PSRS alignment. Anatomic bony landmarks provide a secondary alignment confirmation. Patient positioning systems with translation and rotation capabilities reproduce the planned beam orientations. Verification imaging is performed before each treated field and whenever possible double exposure port views are obtained (see Fig. 12.3).
BENIGN LESIONS
Arteriovenous Malformations
Arteriovenous malformations (AVMs) represent abnormal communications between vessels of disproportionate and unbalanced hydrodynamic stress. The goal of AVM treatment is to completely obliterate the nidus. This eliminates the risk of catastrophic intracranial hemorrhage that is otherwise 2% to 4% per year for these lesions. The fatality rate associated with a single hemorrhage is approximately 10%.1 Resection is the treatment of choice for surgically accessible AVMs. The risks associated with surgical resection are most commonly graded by the Spetzler-Martin five-point scale, which reflects the importance of AVM size, location, and venous drainage.2 Higher grades are associated with increased postoperative morbidity and mortality. In a similar study attempting to design a grading system for AVMs treated by radiosurgery, Pollock et al.3 found patient age, AVM location, and AVM volume to be predictive of obliteration rate. In the event that surgical intervention or embolization of a particular AVM is deemed to be too risky, radiosurgery is the treatment of choice. Common indications for radiosurgery include surgical inaccessibility, expected high probability of surgical morbidity due to large AVM size or eloquent location, or medically inoperable status due to preoperative comorbidity. In addition, some patients simply refuse operative treatment. Photon-based stereotactic radiosurgery using either the Gamma Knife (Elekta; Stockholm, Sweden) or linear accelerator (LINAC) can effectively obliterate relatively small lesions with low treatment-related toxicity.4, 5, 6, 7 Cure, defined as complete obliteration of the vascular lesion, occurs in approximately 80% of cases with smaller AVMs and lower Spetzler-Martin grades. The likelihood of obliteration is related to dose. Obliteration rates increase with time; reirradiation of incompletely responding lesions can raise obliteration rates further without adding significant morbidity.8
Larger unresectable AVMs treated with stereotactic radiosurgery benefit from the use of proton or other heavy charged particle-based radiosurgery. Using charged particle therapy with its finite beam penetration eliminates excess dose beyond the target volume. Both helium and proton radiosurgery have been utilized for this purpose and have indeed enabled safer dose escalation than photon-based therapy. Even at doses equivalent to photons, charged particle therapy would be expected to have fewer long-term sequelae as a result of the absent exit dose.
Much of current knowledge in radiosurgery efficacy and toxic effects derive from early experience with helium ion radiosurgery for AVMs. The University of California,
Berkeley, Lawrence Berkeley Laboratory (UCB-LBL) began treatment of AVM patients with helium ion radiosurgery in 1954.9,10 Patients were largely surgically inaccessible and 25% of patients in the first report had lesions that were >25 mL. Doses of 30 to 45 Gy (RBE) were initially used, delivered in a single fraction. Excellent obliteration rates were achieved with relatively short follow-up and correlated with smaller target size. At 3 years, complete obliteration rates were 100%, 95%, and 70% for lesions of <4 mL, 4 to 25 mL, and >25 mL, respectively. Overall, they achieved a 92% obliteration rate and 4% partial response. Response was a factor of size and dose with smaller lesions having a higher response. In an update, the investigators reported complete obliteration rate at 3 years of 90% to 95% for target volumes of <14 mL. For lesions >14 mL, the obliteration rate was 60% to 70%. The collaborative efforts with Stanford University Medical Center (SUMC) are analyzed separately and among these 56 patients, a 92% complete obliteration rate was achieved at 3 years. Smaller AVMs had distinctly higher obliteration rates, as did those treated to a higher dose. At 1 year, there was a 56% rate of obliteration already with doses between 30 and 45 Gy (RBE). Obliteration rates at 1 year were 20% following treatment of 24 to 28 Gy (RBE) and 17% following 11.5 to 20 Gy (RBE).
Berkeley, Lawrence Berkeley Laboratory (UCB-LBL) began treatment of AVM patients with helium ion radiosurgery in 1954.9,10 Patients were largely surgically inaccessible and 25% of patients in the first report had lesions that were >25 mL. Doses of 30 to 45 Gy (RBE) were initially used, delivered in a single fraction. Excellent obliteration rates were achieved with relatively short follow-up and correlated with smaller target size. At 3 years, complete obliteration rates were 100%, 95%, and 70% for lesions of <4 mL, 4 to 25 mL, and >25 mL, respectively. Overall, they achieved a 92% obliteration rate and 4% partial response. Response was a factor of size and dose with smaller lesions having a higher response. In an update, the investigators reported complete obliteration rate at 3 years of 90% to 95% for target volumes of <14 mL. For lesions >14 mL, the obliteration rate was 60% to 70%. The collaborative efforts with Stanford University Medical Center (SUMC) are analyzed separately and among these 56 patients, a 92% complete obliteration rate was achieved at 3 years. Smaller AVMs had distinctly higher obliteration rates, as did those treated to a higher dose. At 1 year, there was a 56% rate of obliteration already with doses between 30 and 45 Gy (RBE). Obliteration rates at 1 year were 20% following treatment of 24 to 28 Gy (RBE) and 17% following 11.5 to 20 Gy (RBE).
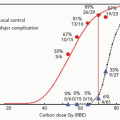
The importance of dose in relation to treatment toxicity was also appreciated by the UCB-LBL group and led to sequential dose reduction overtime as these complications manifested. Patients with history of seizures had a risk of increased seizure activity. Vasogenic edema was radiographically detected in half the number of patients treated with >25 Gy (RBE). Only a subset of these patients became neurologically symptomatic. Most asymptomatic cases resolved without treatment. Most symptomatic vasogenic edema resolved with steroid therapy. No cases of symptomatic vasogenic edema were reported among patients treated with doses <25 Gy (RBE). Similarly, radiation necrosis occurred in 3% of all patients and in no patient receiving <25 Gy (RBE). Dose and volume were the two primary determinants of treatment complications. The collaborative experience with SUMC also demonstrated that lesions in the brainstem, thalamus, and basal ganglia were associated with a higher risk of neurologic complication.
PSRS has been used to treat AVMs at the Harvard Cyclotron Laboratory (HCL) since 1965.11 In an initial report of 75 patients followed for 2 to 16 years, 75% of those who had presented with seizures or severe headaches had symptomatic improvement. Of 62 patients with angiographic follow-up, 20% showed complete AVM obliteration, 56% showed AVM size reduction of >50%, and 13% showed no radiographic changes. Although at first glance, these results suggest that proton radiosurgery may be less effective than helium ion radiosurgery, it should be recognized that the proton series included many patients with large AVMs, often in deep-seated locations, and lower doses of radiation were used as compared to the helium ion series.
Seifert et al.12 reported a limited study of 63 patients referred to a proton treatment facility in the United States but who had both preradiation and postradiation follow-up locally. Similar to the other radiation experiences, patients were largely selected because of surgically inoperable AVMs or patient refusal of surgery. Radiation details are not described but clinical and radiographic results are reported. Among those patients with AVMs no larger than 3 cm in diameter, 76% of them had clinical improvement in symptoms, most commonly of seizures, headaches, or other neurologic symptoms. Twelve percent of patients with small AVMs had progression of symptoms. As AVM size increased, the clinical efficacy of proton radiation dropped and the risk of toxicity increased considerably (33% and 44.5%, respectively, for AVMs >6 cm in diameter). Likewise, 86% of patients with Spetzler-Martin grade 1 or 2 AVMs had clinical improvement compared with only 54% of grade 3 and 24% of grade 4 patients. The obvious limitation of this study was the lack of proton radiation treatment details. Failures or complications could not be evaluated.
A recent report of the PSRS experience for AVMs from the iThemba LABS in the Republic of South Africa reported a 67% obliteration rate for AVM treatment volumes of <14 mL and 43% for larger AVMs at a median follow-up of 62 months.13 These investigators chose their doses judiciously and frequently hypofractionated in two or four fractions for large-volume lesions. Many radiation oncologists and radiation biologists accept an α/β ratio of 3 Gy for AVMs. Some data predict for α/β ratios of 10 Gy or higher. Yet other investigators hypothesize that the α/β ratio may vary within given lesions. The implication of treatment efficacy depending upon the true α/β ratio are discussed by Vernimmen et al.13 Regardless of the true α/β value, these investigators hypothesized that hypofractionation optimized normal tissue tolerance without compromising treatment efficacy. They used minimum doses converted to single fraction equivalents of approximately 15 Gy (RBE) for lesions <14 mL and 10.4 Gy (RBE) for lesions ≥14 mL. Grade 4 toxicity by Radiation Therapy Oncology Group (RTOG) or European Organization for Research and Treatment of Cancer (EORTC) scales was 3%, supporting an accurate prediction of normal brain tissue tolerances.
A smaller experience from Uppsala, Sweden, also reports their results with hypofractionated proton radiosurgery.14 Using two or four fractions to total doses of 20 to 25 Gy, they treated 26 patients and achieved complete obliteration in 7 patients by 3 years. When stratified by AVM size, this data set is generally consistent with other series, with an approximately 70% obliteration rate for AVMs <25 mL in volume and approximately 30% obliteration of AVMs >25 mL in volume. A ≥85% AVM size reduction was seen in 13 of the 26 patients. Seven of nine patients with chronic
seizures had resolution of this symptom. Further response is expected with longer follow-up.
seizures had resolution of this symptom. Further response is expected with longer follow-up.
Toxicity of radiosurgical treatments in a highly curative patient population cannot be overemphasized. Initial data for the UCB-LBL experience has subsequently shaped the approaches used for proton radiosurgery; however, estimation of radiation effects has continued to be imperfect. Kjellberg et al. determined radiosurgical doses based on their early experiences and readily recognized the risk of neurologic complications with increase in dose and AVM size.11 They developed a model to predict complication risks and attempted to select doses based on 1% to 3% isoeffective centile. However, on recent retrospective analysis of the long-term complication rates from the HCL experience of 1,250 patients with available follow-up, there were far greater toxicities seen than expected.15 There was a 1.8% complication rate among those expected to have <1% risk. More notably, there was a 4.7% complication rate for the 128 patients treated at a 1% to 1.8% predictive complication rate, and a 34% rate of complication among the 61 patients treated with an expectant 2% to 2.5% complication risk. Therefore, the efficacy of radiosurgery must be weighed against the apparent toxicities of treatment. The recent analysis of Barker et al.15 has proposed a new model of dose-volume prediction of radiation-induced sequelae that is applied in current dose prescription at the MGH proton facility.
The approach to treating AVMs requires the weighing of several factors. During treatment target definition for radiosurgery, it is important to include the entire nidus and angiographic arterial phase vessels. Partial volume radiosurgery, whether intentional or not, has proved to be inadequate. Shunting of high-pressure blood flow to the remaining vessels increases the risk of hemorrhage. Radiosurgery does not increase the risk of hemorrhage for a treated AVM. Although many believe the risk for hemorrhage is essentially unchanged until the nidus is obliterated, recent data suggests that this risk may actually be reduced by as much as 54% during the latency between radiosurgery and obliteration.16 The gold standard for assessing AVM obliteration is angiography. However, serial magnetic resonance imaging (MRI) is an effective means of following the patient after treatment until the AVM can no longer be seen. Large-volume AVMs that cannot be treated to high doses in single fractions are best treated in a hypofractionated format similar to that described by Vernimmen et al.13
Angiographically Occult Vascular Malformations
Angiographically occult vascular malformations (AOVMs) represent a heterogeneous group of vascular lesions that are not detectable by cerebral angiogram. Of particular importance here are cavernous malformations. The goal of treatment is again to avert catastrophic intracranial bleeding. As with AVMs, the preferred treatment is surgical resection. The role for radiosurgery in surgically inaccessible lesions is less well-defined than for AVMs. In part this relates to the fact that there is no well-defined radiographic endpoint. In the case of AVMs, angiography will confirm that the lesion has been obliterated. In the case of cavernous malformations, which are angiographically occult, this is not feasible. Similarly the lesions do not resolve on MRI or CT scans, even when the treatment appears to have successfully reduced the risk of hemorrhage. Small institutional retrospective studies suggest that Gamma Knife or LINAC-based radiosurgery is safe but efficacy is ill defined.17, 18, 19 Regis et al.17 report on treating 49 patients with symptomatic epilepsy with Gamma Knife radiosurgery and achieved 73% response or reduction or resolution of seizures; however, efficacy as assessed by obliteration rates and reduction of hemorrhage is unknown.
Stanford reported on treatment of 57 patients with AOVMs, 47 with helium ion and the remainder with LINAC-based radiosurgery at a mean dose of 18 Gy (RBE) and 7.5 years follow-up. These investigators documented a higher hemorrhage rate (32%) and higher radiation-related complication rate (10%) than seen with AVMs.20 Although efficacy of treatment according to obliteration rate is unknown, the risk of bleed dramatically falls after the first 3 years from treatment, from 9.4% to 1.6% risk of bleed per year. Similar results are reported with the use of proton beam radiosurgery.21 Among 98 lesions with radiographic findings consistent with cavernous malformations based on imaging and clinical history, an annual risk of hemorrhage was reduced from 17.3% to 4.5% after a 2-year latency period. A 19% complication rate (16% permanent neurologic deficit, 3% mortality) was noted in this series. These studies suggest that radiosurgery reduces the hemorrhagic tendencies of AOVMs after a latency period of 2 to 3 years. However, this reduction is achieved with a significant rate of complication if radiation doses similar to those used for AVM treatment are employed.
Vestibular Schwannomas (Acoustic Neuromas)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree