Herpesviruses are one of the most common groups of pathogens causing central nervous system infections in humans. They mostly cause encephalitis, meningitis, or myelitis in immunocompetent and immunocompromised patients. Children, adults, and the elderly can all be affected. Although contrast-enhanced CT is more widely used for diagnosis, contrast-enhanced MR imaging combined with diffusion-weighted imaging is superior to CT in the detection of early changes and the real extent of the disease, and in assessing prognosis and monitoring response to antiviral treatment. More sophisticated techniques, such as MR spectroscopy and perfusion imaging, can aid in the differential diagnosis of herpesvirus infections from other tumoral, demyelinating, and ischemic processes.
Human herpesviruses (HHV) are one the most common causes of human viral infections worldwide. This article reviews the pathogenesis of herpetic diseases and the diagnostic approaches to the common pathologies of the HHV family in children and adults. Intrauterine and neonatal herpesvirus infections are beyond the scope of this article.
The HHV family consists of a large group of DNA viruses, which are categorized on the basis of their molecular and biologic properties as alpha viruses, including herpes simplex virus type 1 (HSV-1), type 2 (HSV-2), B virus, and varicella zoster virus (VZV); beta viruses, including cytomegalovirus (CMV), HHV type 6 (HHV-6), and type 7 (HHV-7); and gamma viruses, including Epstein-Barr virus (EBV) and HHV type 8 (HHV-8). The herpesvirus family has an icosahedral nucleocapsid, consisting of 162 capsomeres arranged around a linear, double-stranded DNA core; a proteinaceous tegument; and a proteolipid envelope. Each of these has different surface glycoproteins in different herpesviruses, which give the distinctive characteristics of each virus . Humans are the sole reservoirs for them. Epidemiologically, HHV can cause infections in infants, children, or adults. The vectors usually spread by direct contact with the virus-infected secretions of other humans, and rarely by blood transfusion and tissue transplantation .
Mechanism of the herpesvirus infections
The skin, conjunctiva, and mucosa of the oropharynx or genitalia are the primary entry sites for HHV. Following replication in the inoculation site, they usually cause a hematogenous spread, called a viremia, to distant tissues . All herpesviruses show some kind of neurotropism, either by hematogenous spread or neuronal transmission . The neurovirulence of herpesviruses is mediated by the thymidine kinase gene and the termini of the L component . The γ 1 34.5 gene is required for the replication of herpesviruses in brain tissue and prevents apoptosis of infected neuronal cells . Genetically engineered HSV virions lacking the γ 1 34.5 gene are being investigated for brain tumor treatment . The hematogenous spread to the central nervous system (CNS) is thought to be the mechanism of neonatal HSV-1, HSV-2, CMV, EBV, HSV-6, and HSV-7 infections, either by diffusing through the blood–brain barrier or by infecting the endothelial cells of blood vessels in the brain . On the other hand, neuronal transmission is thought to be the mechanism of adult HSV-1, VZV, and B virus infections, by retrograde transportation of the viruses through the axons of the sensory and sympathetic peripheral neurons to their nuclei . Particularly in immunocompromised patients, the two mechanisms can be concurrently responsible for CNS infection .
The most important characteristic of HHV is its latency in neuronal tissue before being reactivated by a stimulus . During this latency period, the viral genome is transcriptionally silent, except for a single region encoding the latency-associated transcript, which has been shown to affect apoptosis, and inhibits initial dendritic growth and induces dendritic retraction in sympathetic neurons . Putative sites of cellular latency include the neural ganglia for HSV and VZV, epithelial cells for EBV, leukocytes or endothelial cells for CMV, and lymphocytes for HHV-6 and HHV-7 . Events such as physical or emotional stress, fever, ultraviolet light, and tissue damage can reactivate the latent virus, and, in some cases, can cause exacerbation of herpetic CNS disease . In at least one third of patients, in situ reactivation of the virus (latent in brain tissue) appears to be a more likely scenario than retrograde spread along a cranial nerve . Animal studies support the hypothesis of stress-induced reactivation of the virus, mediated by the glucocorticoid receptors, and CNS inflammation during stress mediated by N-methyl-D-aspartate receptors can promote the development of herpes simplex encephalitis (HSE) , but HSV-1 reactivates from a small fraction of latently infected neurons . Additionally, herpesviruses in the latent period can be reactivated once HIV has damaged the cellular immune system and can subsequently affect disease progression and survival in patients who have AIDS by causing an opportunistic disease. The inhibition of herpesvirus reactivation may provide a clinical and survival benefit to these individuals .
The incidence and clinical manifestation of herpetic CNS infections are related to complex interactions among the virus strain, the host cells, and the immune status of the infected host , and may vary considerably between immunocompetent and immunocompromised individuals, and in young children . The spectrum of the herpetic neurologic diseases, ranging from febrile seizures, to neuritis, polyradiculitis, or myelitis, to aseptic or recurrent meningitis, or ventriculitis, to severe leukoencephalitis or necrotizing encephalitis, increased dramatically over the last decade because of improved applications of immunohistochemistry, in situ hybridization, and polymerase chain reaction (PCR) to cerebrospinal fluid (CSF) and brain tissue . Possible associations have also been reported between HHV-6 and multiple sclerosis ; HSV-1 and Alzheimer’s disease ; lymphoma and EBV in immunocompromised individuals ; and among EBV, CMV, HHV-6, and chronic fatigue syndrome .
Herpes simplex virus type 1
HSV is the most common cause of acute fatal sporadic encephalitis in human beings . It usually produces focal encephalitis rather than diffuse or nonfocal disease . The actual vector of HSE is HSV-1 in most patients (>90%) and HSV-2 in the remainder . HSE was thought to account for 10% to 20% of all viral encephalitis, with an annual incidence of 1/250,000 to 500,000, before the introduction of West Nile virus encephalitis . Mainly, HSE is the disease of children and the elderly. It has been reported that approximately one third of HSE cases, in patients ranging in age from 6 months to 20 years, are the consequence of primary infection, and approximately one half of cases in patients older than 50 years are the consequence of reactivation of HSV . HSE shows no age, sex, or seasonal predilection but, especially with brainstem involvement, may be more common and extensive in immunocompromised patients than in immunocompetent ones . The mortality rate of HSE exceeds 70% in patients who had no or incomplete treatment and only 2.5% of all patients with confirmed disease (9.1% of survivors) returned to normal function after recovering from their illness .
HSE typically presents with a broad range of nonspecific signs and symptoms of focal encephalopathy, including headache, fever, nuchal rigidity, changes in personality and mental status, focal or generalized seizures, and acutely decreased level of consciousness, with focal neurologic signs such as weakness, sensory abnormalities, aphasia, visual field defects, or cranial nerve palsies (see Refs. ). Although none of these findings are diagnostic for HSE, this disease should be considered in a patient with a progressively deteriorating level of consciousness, fever, abnormal CSF indices, and focal neurologic findings, in the absence of other causes . Of 432 patients who had encephalitis, HSV was isolated from brain tissue in 195 (45%) . In its chronic stage, HSE can cause depressive symptoms, somnambulism, long-term memory disturbance, and intracerebral hematoma involving the initial encephalitic area .
The CSF findings of HSE, such as pleocytosis, proteinosis, and absence of other bacterial and fungal pathogens, are also nonspecific. The isolation of the HSV from the CSF is also rare, because anti-HSV antibodies in the CSF occur later in the natural course of the disease . Early in the disease, focal electroencephalographic (EEG) findings, characterized by spike and slow-wave activity, and periodic lateralized epileptiform discharges, which arise from the temporal lobe, may suggest an HSE diagnosis, but the abnormal electric activity usually spreads to the contralateral temporal lobe as the disease evolves . The sensitivity of the EEG is approximately 84%, but the specificity is only 32.5% . The most sensitive and specific means of diagnosis is the isolation of HSV from tissue obtained at brain biopsy, with a sensitivity of 96% and a specificity of 99% . Recently, the PCR technique, which is an amplification method of detecting HSV-DNA in the CSF, has replaced routine brain biopsy for diagnostic purposes , and has widely prevented the acute or chronic complications of biopsies (in approximately 3%), including the potential of progressing acute illness or causing chronic seizure disorders . PCR has a sensitivity of 98%, a specificity of 94%, a positive predictive value of 95%, and a negative predictive value of 98%, similar to the values of brain biopsy . A single negative PCR result does not exclude the possibility of HSV, particularly if the CSF was obtained during the first 72 hours after onset of clinical symptoms, and it should be repeated during the natural course of the disease . The virus load in the CSF monitoring by real-time PCR detection of HSV DNA, the decreased level of consciousness, and the presence of abnormal radiologic findings are statistically well correlated with poor neurologic outcome .
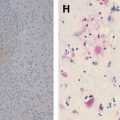
In the acute stage, HSE results in acute inflammation, congestion, or petechial hemorrhage, particularly in the temporal lobe and adjacent meninges, followed by frank hemorrhagic necrosis and liquefaction, in approximately 2 weeks . The diffuse perivascular subarachnoid mononuclear cell infiltrate, glial nodules, satellitosis, neuronophagia, and Cowdry type A inclusions within involved cells are identified in the subacute stage of HSE .
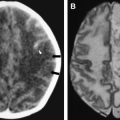
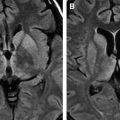
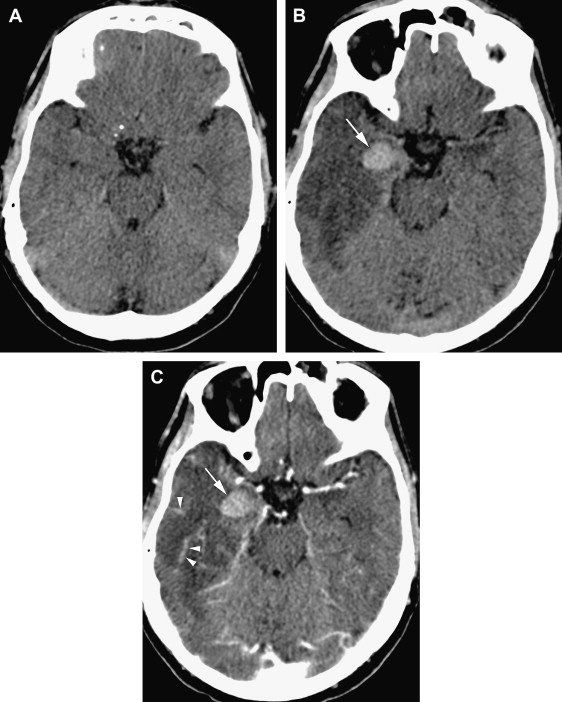
MR imaging, especially when combined with diffusion-weighted (DW) imaging, is more sensitive than CT in the detection of early brain changes in HSE . Initial CT images are normal in up to 25% of HSE patients and become positive only after the second week . Occasionally, subsequent CT shows low-density areas with mass effect localized to the temporal lobe, which can progress to radiolucent or hemorrhagic lesions involving both temporal lobes, particularly late in the course of the disease . A patchy parenchymal or meningeal pattern of contrast enhancement is rarely detected before the second week of clinical symptoms on CT ( Fig. 1 ) . In the MR imaging of HSE, lesions are usually isointense or hypointense on T1-weighted (T1W) and hyperintense on T2-weighted (T2W) images and usually do not enhance. These early changes, which characteristically involve the medial and inferior aspects of the temporal lobe extending up into the insula within the first 48 hours on T2W images or fluid-attenuated inversion recovery (FLAIR) images, are consistent with edema and inflammation ( Figs. 2 A, B and 3 A, B) . In the early phase of hemorrhage, both CT and MR imaging are demonstrable. Following the acute stage (>1 week), MR imaging is superior to CT in detecting hemorrhagic changes, because of characteristic signal properties defined by the phase of hemoglobin degradation products on T1W and T2W images. Gradient-echo T2W images are also more prone to detecting blood degradation products than conventional T1W and T2W spin-echo images. Subcortical low intensity areas on T2W imaging, FLAIR, and DW imaging, reported in some other meningitis, viral encephalitis, and leptomeningeal metastasis, is uncommon in HSE .
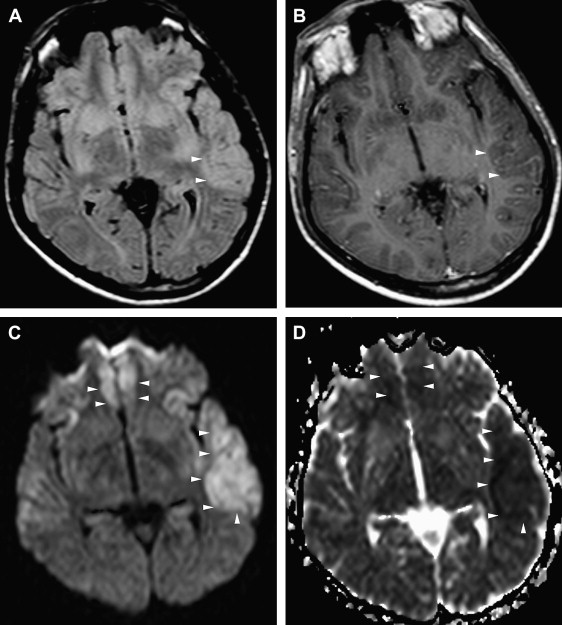
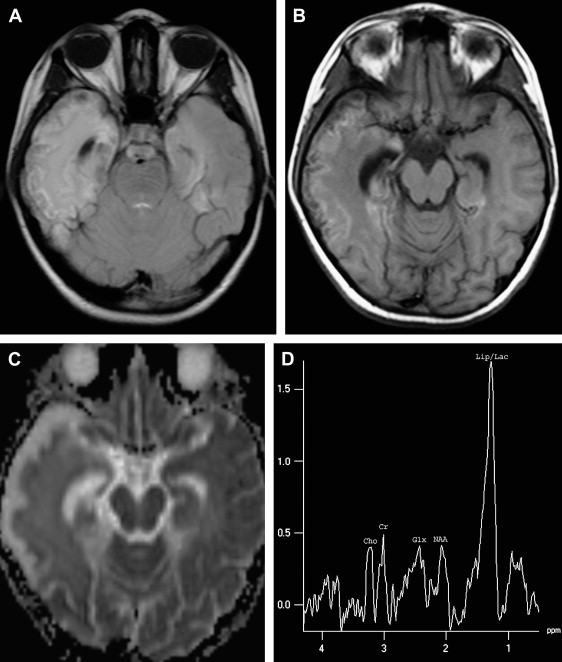
DW imaging is more sensitive than T2W or FLAIR imaging in the early detection of necrotizing encephalitis (see Refs. ). In the early stage of the disease, two different diffusional changes can occur. The first is the decrease in water diffusion due to cytotoxic edema, seen as hyperintense on trace DW imaging and hypointense on apparent diffusion coefficient (ADC) maps ( Fig. 2 C, D), and usually reflecting irreversible neuronal damage undergoing necrosis and poor outcome in patients who have HSE . The second is the increase in water diffusion due to vasogenic edema, seen as high signal areas on trace DW imaging and ADC maps ( Fig. 3 C, D), representing more reversible changes following adequate treatment and a more favorable outcome . These two early changes usually take place concurrently in most patients and seldom occur individually. These initial changes, especially the latter, should be differentiated from the chronic diffusional changes seen in the late stage of the disease. The evolving spectrum of pathologic changes, from cytotoxic edema to cellular lysis and necrosis during the natural course of the disease, causes a change in free water diffusion, from a decrease to an increase, within 14 days of symptom onset, similar to the pseudonormalization period of ischemic lesions, and results in abnormal high signal on T2W and DW images ( Fig. 4 A, C) . Necrotic and hemorrhagic changes are clearly visible on T1W images ( Fig. 4 B). DW imaging may also aid in distinguishing herpetic lesions from infiltrative temporal lobe tumors because the ADCs of herpes lesions are lower than normal brain parenchyma (ranging from 0.48 to 0.66), whereas the ADCs of various tumors are elevated or in the normal range .
Different enhancement patterns, such as gyral, meningeal, diffuse, or ring-like, have been reported in up to 50% of patients following initiation of T2 signal abnormality . HSE produces mostly superficial gray matter disease, changing signal intensity, and a breakdown of the blood–brain barrier to produce contrast enhancement in a cortical gyral pattern (see Fig. 3 B) . This enhancement pattern may lag behind the onset of signs and symptoms or may be suppressed by steroid medications; thus, the absence of cortical gyral enhancement does not exclude encephalitis . In the early course of HSE, diffuse enhancement helps to differentiate it from acute infarction, which has a DW imaging pattern similar to HSE. Enhancement is typically not diffuse in infarcts and only becomes prominent in the subacute stage (>2 weeks). The use of magnetization transfer prepulse can increase the sensitivity of contrast-enhanced images in the delineation of meningeal and parenchymal enhancement .
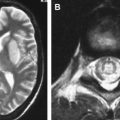
In adults, HSE typically involves the anterior and medial aspects of the temporal and the orbital frontal lobes, but one side is usually more severely affected. This classic type of involvement indicates the probable mechanism of intracranial spread along the small meningeal branches of the trigeminal nerve from the trigeminal ganglion . The infectious process may extend to the insular, angular, and posterior occipital cortexes or to the higher frontal and parietal cortexes ( Fig. 5 ) . Although the lentiform nucleus tends to be spared , basal ganglia can be affected, with simultaneous temporal lobe involvement . Extratemporal involvement occurs in up to 55% of patients, including the frontal, parietal, occipital lobes, the limbic system, the cingulate gyrus, the brain stem, and the thalami (see Refs. ). In 15% of HSE patients, pure extratemporal involvement can be seen . Cingulate gyrus is usually involved by way of the anatomic connection to the affected ipsilateral hippocampus , and pons and mesencephalon by retrograde viral transmission along the cisternal portion of the trigeminal nerve to the brainstem . Besides these characteristic radiologic patterns, diffuse brain involvement has also been reported in HSE .
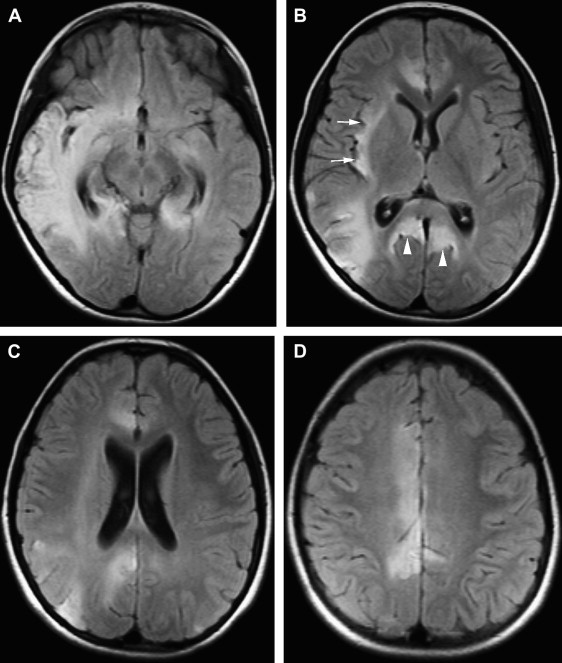
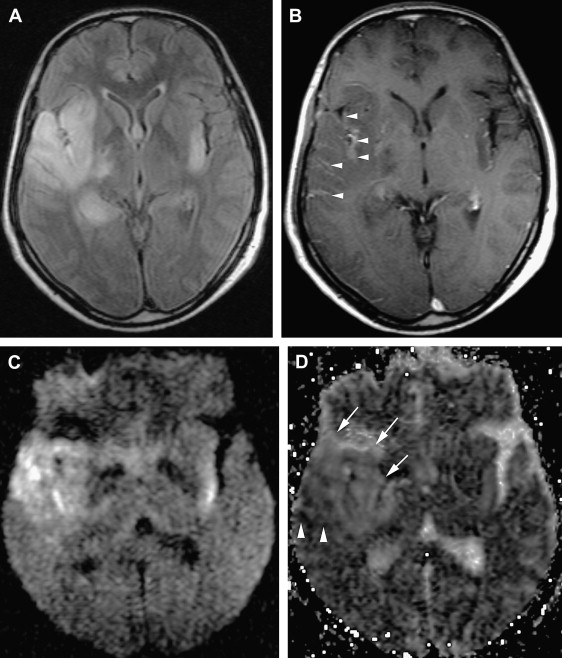
The involvement pattern of HSE in neonates and young children often differs from the adult frontal-temporal pattern ( Fig. 6 ). In young children, lesions are more diffuse or multifocal, and usually bilateral and more symmetric, than those seen in adults. Enhanced thickened cortical or white matter lesions throughout all lobes of the cerebral hemispheres, the insulae, and the thalami, corresponding to the vascular distribution pattern of the anterior, middle, and posterior cerebral arteries and their perforating branches, suggest a hematogenous route for the spread of the virus into the CNS in young children . This involvement pattern of HSV-1 encephalitis is significantly different from the periventricular white matter lesions and meningeal enhancement seen in neonatal HSV-2 encephalitis, which has a worse prognosis . HSE during the first few years of life can be complicated by chronic granulomatous inflammation with mineralization, which causes intractable epilepsy, requiring neurosurgery .
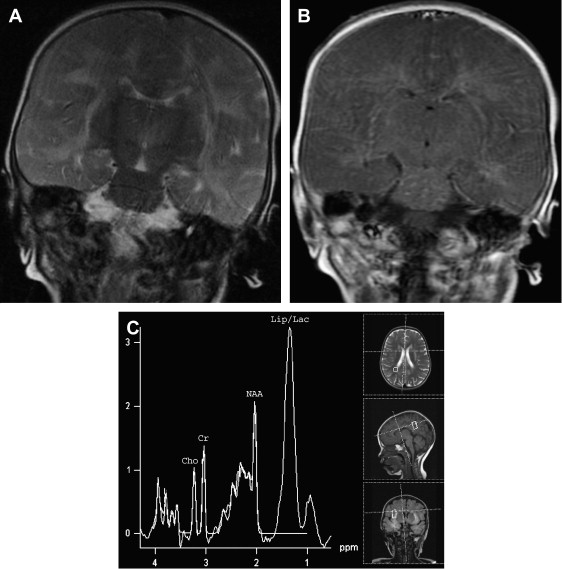
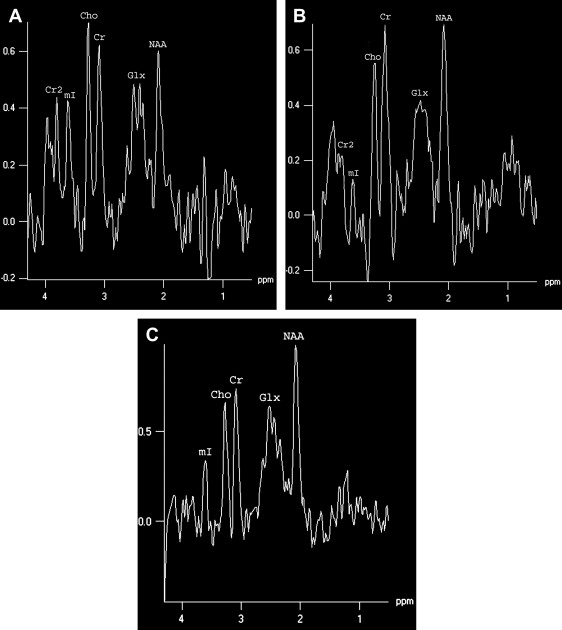
MR spectroscopy studies have revealed that N-acetyl aspartate (NAA) level and NAA/creatine ratio decrease during an acute course of HSE and recover step by step to normal limits within a year ( Fig. 7 ) because of the reversal of the loss of neuronal integrity or function during the acute inflammatory process . Macrophage infiltration, prominent in the acute stage of HSE, can cause an increase in choline level and choline/creatine ratio and may resemble an infiltrative tumoral process . An associated increase in myo-inositol and myo-inositol/creatine ratio due to marked gliosis may be suggestive of infection, but the same spectral pattern can also be identified in low-grade astrocytomas . Lactate and lipid, and sometimes glutamate-glutamine complex (Glx), may also increase in the acute stage of necrotizing encephalitis ( Fig. 4 D; see Fig. 6 C), but this increase is less prominent in chronic encephalitis, such as Rasmussen’s encephalitis . Multivoxel MR spectroscopy studies of the whole brain show that some kind of spectral abnormalities can also be seen in contralateral normal-appearing parenchyma, indicating global involvement of the brain in HSE .
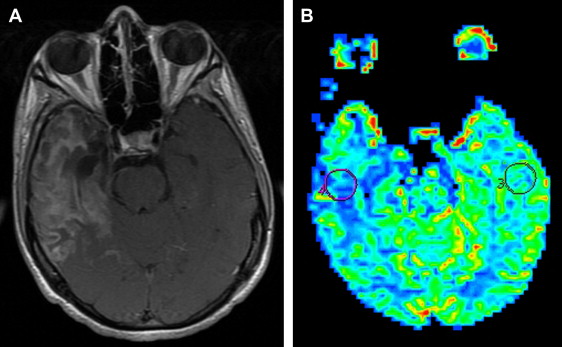
CT perfusion studies demonstrate an abnormal increase of blood flow in the affected temporoparietal cortex at an early stage . The technetium-99m-pertechnetate scans show a moderate uptake caused by disruption of the blood–brain barrier in the early stage but fail to show this if the blood–brain barrier is intact . Focal hyperactivity of affected brain regions in technetium-99m-hexamethyl propylene amine oxime (HMPAO) or 123 I-iodoamphetamine scans has been considered a hallmark finding of acute HSE and matches the hyperintense signal seen on MR images . Although an increased uptake of technetium-99m-HMPAO was detected in the affected brain areas, the uptake of technetium-99m-ethyl cysteinate dimmer was reduced in corresponding areas . Discordance in the uptake of both blood flow tracers not only reflects increased perfusion in HSE but also disruption of the cell membrane and intracellular metabolism, changing tracer kinetics . The authors’ experience and the literature show that HSE usually presents with lower rCBV and rCBF values than normal parenchyma in MR perfusion studies ( Fig. 8 ). Perfusion imaging is mainly useful to differentiate the infection, which demonstrates decreased perfusion, from the tumoral infiltration, which is characterized by increased perfusion. Histopathologically matched data are not available; however, the decreased perfusion areas in HSE are probably caused by alterations in blood–brain barrier function or decreased vascularity during the acute infectious stage, and by reduced metabolism caused by neuronal death during the late course of the disease. Furthermore, in vivo bioluminescence imaging studies in living mice have shown promising results in the elucidation of herpes-associated disease mechanisms and have provided noninvasive, real-time monitoring of hematogenous infection .
Although asymmetric temporal lobe involvement is typical of HSE, similar findings can be identified in the infiltrative tumoral process, cerebral infraction, paraneoplastic limbic encephalopathy, lupus erythematosus, Hurst hemorrhagic leukoencephalitis, Japanese encephalitis, and meningovascular neurosyphilis . Even though isolated temporal lobe involvement is rarely seen in these pathologies, they should be kept in the differential diagnosis of patients, especially those who do not respond well to antiviral therapy. Bilateral temporal lobe involvement and striking chronic atrophic changes are more prominent in HSE than in the others. In the acute stage, the MR spectroscopy findings of encephalitis and gliomas are almost the same, whereas the infarctions reveal high lactate levels, differentiating the disorder from the other two pathologies . The differentiation of encephalitis and gliomas based on MR findings could reliably be made with short-time follow-up MR examinations; gliomas tend to grow, in contrast to encephalitis, which shows regression . Additionally, perfusion imaging can help to differentiate HSE, characterized by low rCBV values, from infiltrative glial tumor, usually represented with high rCBV values, but sometimes low-grade gliomas also have lower rCBV values than normal parenchyma . In Japanese encephalitis, temporal lobe involvement mainly includes the hippocampus, sparing the rest of the temporal lobe, and the concurrent involvement of the thalami, substantia nigra, and basal ganglia allows its differentiation from HSE .
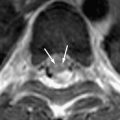
HSV-1 can also infect other areas of the CNS concomitantly, with or without encephalitis. Cranial nerve involvement, revealed on contrast-enhanced MR studies, can occur because of reactivation of the virus from the nerve ganglion, but its real incidence is probably underestimated. Fifth or eighth nerve neuritis with simultaneous rhombencephalitis and mesencephalitis; optic nerve enlargement with involvement of the left lateral geniculate body and left occipital lobe; or seventh nerve involvement causing idiopathic Bell’s palsy ( Fig. 9 ) have been reported . The results of studies about HSV encephalomyeloradiculitis or myelitis have shown that HSV-2 causes HSV myelitis more frequently than HSV-1 in immunocompetent adults . HSV myelitis is typically represented as intramedullary hyperintensity of the spinal cord on T2W images, with a corresponding localized dermatomal rash and neurologic symptoms .
Current research also supports the hypothesis that HSV-1 infection of the CNS, although not the cause of Alzheimer’s disease, may initiate the disease, potentially predisposing individuals to increased inflammation, neuritic plaque, neurofibrillary tangle formation, and beta-amyloid deposition, but further research is required to clarify the role of this virus in this chronic disease .
Herpes simplex virus type 1
HSV is the most common cause of acute fatal sporadic encephalitis in human beings . It usually produces focal encephalitis rather than diffuse or nonfocal disease . The actual vector of HSE is HSV-1 in most patients (>90%) and HSV-2 in the remainder . HSE was thought to account for 10% to 20% of all viral encephalitis, with an annual incidence of 1/250,000 to 500,000, before the introduction of West Nile virus encephalitis . Mainly, HSE is the disease of children and the elderly. It has been reported that approximately one third of HSE cases, in patients ranging in age from 6 months to 20 years, are the consequence of primary infection, and approximately one half of cases in patients older than 50 years are the consequence of reactivation of HSV . HSE shows no age, sex, or seasonal predilection but, especially with brainstem involvement, may be more common and extensive in immunocompromised patients than in immunocompetent ones . The mortality rate of HSE exceeds 70% in patients who had no or incomplete treatment and only 2.5% of all patients with confirmed disease (9.1% of survivors) returned to normal function after recovering from their illness .
HSE typically presents with a broad range of nonspecific signs and symptoms of focal encephalopathy, including headache, fever, nuchal rigidity, changes in personality and mental status, focal or generalized seizures, and acutely decreased level of consciousness, with focal neurologic signs such as weakness, sensory abnormalities, aphasia, visual field defects, or cranial nerve palsies (see Refs. ). Although none of these findings are diagnostic for HSE, this disease should be considered in a patient with a progressively deteriorating level of consciousness, fever, abnormal CSF indices, and focal neurologic findings, in the absence of other causes . Of 432 patients who had encephalitis, HSV was isolated from brain tissue in 195 (45%) . In its chronic stage, HSE can cause depressive symptoms, somnambulism, long-term memory disturbance, and intracerebral hematoma involving the initial encephalitic area .
The CSF findings of HSE, such as pleocytosis, proteinosis, and absence of other bacterial and fungal pathogens, are also nonspecific. The isolation of the HSV from the CSF is also rare, because anti-HSV antibodies in the CSF occur later in the natural course of the disease . Early in the disease, focal electroencephalographic (EEG) findings, characterized by spike and slow-wave activity, and periodic lateralized epileptiform discharges, which arise from the temporal lobe, may suggest an HSE diagnosis, but the abnormal electric activity usually spreads to the contralateral temporal lobe as the disease evolves . The sensitivity of the EEG is approximately 84%, but the specificity is only 32.5% . The most sensitive and specific means of diagnosis is the isolation of HSV from tissue obtained at brain biopsy, with a sensitivity of 96% and a specificity of 99% . Recently, the PCR technique, which is an amplification method of detecting HSV-DNA in the CSF, has replaced routine brain biopsy for diagnostic purposes , and has widely prevented the acute or chronic complications of biopsies (in approximately 3%), including the potential of progressing acute illness or causing chronic seizure disorders . PCR has a sensitivity of 98%, a specificity of 94%, a positive predictive value of 95%, and a negative predictive value of 98%, similar to the values of brain biopsy . A single negative PCR result does not exclude the possibility of HSV, particularly if the CSF was obtained during the first 72 hours after onset of clinical symptoms, and it should be repeated during the natural course of the disease . The virus load in the CSF monitoring by real-time PCR detection of HSV DNA, the decreased level of consciousness, and the presence of abnormal radiologic findings are statistically well correlated with poor neurologic outcome .
In the acute stage, HSE results in acute inflammation, congestion, or petechial hemorrhage, particularly in the temporal lobe and adjacent meninges, followed by frank hemorrhagic necrosis and liquefaction, in approximately 2 weeks . The diffuse perivascular subarachnoid mononuclear cell infiltrate, glial nodules, satellitosis, neuronophagia, and Cowdry type A inclusions within involved cells are identified in the subacute stage of HSE .
MR imaging, especially when combined with diffusion-weighted (DW) imaging, is more sensitive than CT in the detection of early brain changes in HSE . Initial CT images are normal in up to 25% of HSE patients and become positive only after the second week . Occasionally, subsequent CT shows low-density areas with mass effect localized to the temporal lobe, which can progress to radiolucent or hemorrhagic lesions involving both temporal lobes, particularly late in the course of the disease . A patchy parenchymal or meningeal pattern of contrast enhancement is rarely detected before the second week of clinical symptoms on CT ( Fig. 1 ) . In the MR imaging of HSE, lesions are usually isointense or hypointense on T1-weighted (T1W) and hyperintense on T2-weighted (T2W) images and usually do not enhance. These early changes, which characteristically involve the medial and inferior aspects of the temporal lobe extending up into the insula within the first 48 hours on T2W images or fluid-attenuated inversion recovery (FLAIR) images, are consistent with edema and inflammation ( Figs. 2 A, B and 3 A, B) . In the early phase of hemorrhage, both CT and MR imaging are demonstrable. Following the acute stage (>1 week), MR imaging is superior to CT in detecting hemorrhagic changes, because of characteristic signal properties defined by the phase of hemoglobin degradation products on T1W and T2W images. Gradient-echo T2W images are also more prone to detecting blood degradation products than conventional T1W and T2W spin-echo images. Subcortical low intensity areas on T2W imaging, FLAIR, and DW imaging, reported in some other meningitis, viral encephalitis, and leptomeningeal metastasis, is uncommon in HSE .
DW imaging is more sensitive than T2W or FLAIR imaging in the early detection of necrotizing encephalitis (see Refs. ). In the early stage of the disease, two different diffusional changes can occur. The first is the decrease in water diffusion due to cytotoxic edema, seen as hyperintense on trace DW imaging and hypointense on apparent diffusion coefficient (ADC) maps ( Fig. 2 C, D), and usually reflecting irreversible neuronal damage undergoing necrosis and poor outcome in patients who have HSE . The second is the increase in water diffusion due to vasogenic edema, seen as high signal areas on trace DW imaging and ADC maps ( Fig. 3 C, D), representing more reversible changes following adequate treatment and a more favorable outcome . These two early changes usually take place concurrently in most patients and seldom occur individually. These initial changes, especially the latter, should be differentiated from the chronic diffusional changes seen in the late stage of the disease. The evolving spectrum of pathologic changes, from cytotoxic edema to cellular lysis and necrosis during the natural course of the disease, causes a change in free water diffusion, from a decrease to an increase, within 14 days of symptom onset, similar to the pseudonormalization period of ischemic lesions, and results in abnormal high signal on T2W and DW images ( Fig. 4 A, C) . Necrotic and hemorrhagic changes are clearly visible on T1W images ( Fig. 4 B). DW imaging may also aid in distinguishing herpetic lesions from infiltrative temporal lobe tumors because the ADCs of herpes lesions are lower than normal brain parenchyma (ranging from 0.48 to 0.66), whereas the ADCs of various tumors are elevated or in the normal range .
Different enhancement patterns, such as gyral, meningeal, diffuse, or ring-like, have been reported in up to 50% of patients following initiation of T2 signal abnormality . HSE produces mostly superficial gray matter disease, changing signal intensity, and a breakdown of the blood–brain barrier to produce contrast enhancement in a cortical gyral pattern (see Fig. 3 B) . This enhancement pattern may lag behind the onset of signs and symptoms or may be suppressed by steroid medications; thus, the absence of cortical gyral enhancement does not exclude encephalitis . In the early course of HSE, diffuse enhancement helps to differentiate it from acute infarction, which has a DW imaging pattern similar to HSE. Enhancement is typically not diffuse in infarcts and only becomes prominent in the subacute stage (>2 weeks). The use of magnetization transfer prepulse can increase the sensitivity of contrast-enhanced images in the delineation of meningeal and parenchymal enhancement .
In adults, HSE typically involves the anterior and medial aspects of the temporal and the orbital frontal lobes, but one side is usually more severely affected. This classic type of involvement indicates the probable mechanism of intracranial spread along the small meningeal branches of the trigeminal nerve from the trigeminal ganglion . The infectious process may extend to the insular, angular, and posterior occipital cortexes or to the higher frontal and parietal cortexes ( Fig. 5 ) . Although the lentiform nucleus tends to be spared , basal ganglia can be affected, with simultaneous temporal lobe involvement . Extratemporal involvement occurs in up to 55% of patients, including the frontal, parietal, occipital lobes, the limbic system, the cingulate gyrus, the brain stem, and the thalami (see Refs. ). In 15% of HSE patients, pure extratemporal involvement can be seen . Cingulate gyrus is usually involved by way of the anatomic connection to the affected ipsilateral hippocampus , and pons and mesencephalon by retrograde viral transmission along the cisternal portion of the trigeminal nerve to the brainstem . Besides these characteristic radiologic patterns, diffuse brain involvement has also been reported in HSE .
The involvement pattern of HSE in neonates and young children often differs from the adult frontal-temporal pattern ( Fig. 6 ). In young children, lesions are more diffuse or multifocal, and usually bilateral and more symmetric, than those seen in adults. Enhanced thickened cortical or white matter lesions throughout all lobes of the cerebral hemispheres, the insulae, and the thalami, corresponding to the vascular distribution pattern of the anterior, middle, and posterior cerebral arteries and their perforating branches, suggest a hematogenous route for the spread of the virus into the CNS in young children . This involvement pattern of HSV-1 encephalitis is significantly different from the periventricular white matter lesions and meningeal enhancement seen in neonatal HSV-2 encephalitis, which has a worse prognosis . HSE during the first few years of life can be complicated by chronic granulomatous inflammation with mineralization, which causes intractable epilepsy, requiring neurosurgery .
MR spectroscopy studies have revealed that N-acetyl aspartate (NAA) level and NAA/creatine ratio decrease during an acute course of HSE and recover step by step to normal limits within a year ( Fig. 7 ) because of the reversal of the loss of neuronal integrity or function during the acute inflammatory process . Macrophage infiltration, prominent in the acute stage of HSE, can cause an increase in choline level and choline/creatine ratio and may resemble an infiltrative tumoral process . An associated increase in myo-inositol and myo-inositol/creatine ratio due to marked gliosis may be suggestive of infection, but the same spectral pattern can also be identified in low-grade astrocytomas . Lactate and lipid, and sometimes glutamate-glutamine complex (Glx), may also increase in the acute stage of necrotizing encephalitis ( Fig. 4 D; see Fig. 6 C), but this increase is less prominent in chronic encephalitis, such as Rasmussen’s encephalitis . Multivoxel MR spectroscopy studies of the whole brain show that some kind of spectral abnormalities can also be seen in contralateral normal-appearing parenchyma, indicating global involvement of the brain in HSE .
CT perfusion studies demonstrate an abnormal increase of blood flow in the affected temporoparietal cortex at an early stage . The technetium-99m-pertechnetate scans show a moderate uptake caused by disruption of the blood–brain barrier in the early stage but fail to show this if the blood–brain barrier is intact . Focal hyperactivity of affected brain regions in technetium-99m-hexamethyl propylene amine oxime (HMPAO) or 123 I-iodoamphetamine scans has been considered a hallmark finding of acute HSE and matches the hyperintense signal seen on MR images . Although an increased uptake of technetium-99m-HMPAO was detected in the affected brain areas, the uptake of technetium-99m-ethyl cysteinate dimmer was reduced in corresponding areas . Discordance in the uptake of both blood flow tracers not only reflects increased perfusion in HSE but also disruption of the cell membrane and intracellular metabolism, changing tracer kinetics . The authors’ experience and the literature show that HSE usually presents with lower rCBV and rCBF values than normal parenchyma in MR perfusion studies ( Fig. 8 ). Perfusion imaging is mainly useful to differentiate the infection, which demonstrates decreased perfusion, from the tumoral infiltration, which is characterized by increased perfusion. Histopathologically matched data are not available; however, the decreased perfusion areas in HSE are probably caused by alterations in blood–brain barrier function or decreased vascularity during the acute infectious stage, and by reduced metabolism caused by neuronal death during the late course of the disease. Furthermore, in vivo bioluminescence imaging studies in living mice have shown promising results in the elucidation of herpes-associated disease mechanisms and have provided noninvasive, real-time monitoring of hematogenous infection .
Although asymmetric temporal lobe involvement is typical of HSE, similar findings can be identified in the infiltrative tumoral process, cerebral infraction, paraneoplastic limbic encephalopathy, lupus erythematosus, Hurst hemorrhagic leukoencephalitis, Japanese encephalitis, and meningovascular neurosyphilis . Even though isolated temporal lobe involvement is rarely seen in these pathologies, they should be kept in the differential diagnosis of patients, especially those who do not respond well to antiviral therapy. Bilateral temporal lobe involvement and striking chronic atrophic changes are more prominent in HSE than in the others. In the acute stage, the MR spectroscopy findings of encephalitis and gliomas are almost the same, whereas the infarctions reveal high lactate levels, differentiating the disorder from the other two pathologies . The differentiation of encephalitis and gliomas based on MR findings could reliably be made with short-time follow-up MR examinations; gliomas tend to grow, in contrast to encephalitis, which shows regression . Additionally, perfusion imaging can help to differentiate HSE, characterized by low rCBV values, from infiltrative glial tumor, usually represented with high rCBV values, but sometimes low-grade gliomas also have lower rCBV values than normal parenchyma . In Japanese encephalitis, temporal lobe involvement mainly includes the hippocampus, sparing the rest of the temporal lobe, and the concurrent involvement of the thalami, substantia nigra, and basal ganglia allows its differentiation from HSE .
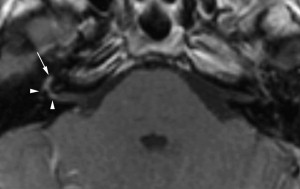
HSV-1 can also infect other areas of the CNS concomitantly, with or without encephalitis. Cranial nerve involvement, revealed on contrast-enhanced MR studies, can occur because of reactivation of the virus from the nerve ganglion, but its real incidence is probably underestimated. Fifth or eighth nerve neuritis with simultaneous rhombencephalitis and mesencephalitis; optic nerve enlargement with involvement of the left lateral geniculate body and left occipital lobe; or seventh nerve involvement causing idiopathic Bell’s palsy ( Fig. 9 ) have been reported . The results of studies about HSV encephalomyeloradiculitis or myelitis have shown that HSV-2 causes HSV myelitis more frequently than HSV-1 in immunocompetent adults . HSV myelitis is typically represented as intramedullary hyperintensity of the spinal cord on T2W images, with a corresponding localized dermatomal rash and neurologic symptoms .