35
Central Venous Access
Modern medical care has rapidly expanded the need for the placement of catheters into the central venous circulation for long periods. Long-term central venous access is commonly used for hemodialysis, plasmapheresis, chemotherapy, long-term antibiotic therapy, blood drawing, parenteral nutrition, and analgesics. Outpatient dialysis centers, ambulatory chemotherapy, hospices, home health care, and other commercial enterprises have grown dramatically as an indirect result of these devices.
Broviac and Hickman described the first soft long-term right atrial silicone catheters in 1973 and 1979, respectively.1,2 The first subcutaneous port was reported by Niederhuber in 1982.3 Historically these devices have been placed by surgeons within an operating room. The traditional role for radiologists was to be involved in preoperative venography, catheter relocation, and intravascular catheter fragment retrieval. More recently, the interventional radiologist’s role has expanded to include complex access cases and the development of an independent venous access service where they function as the primary operator in the placement and management of all long-term devices.4,5
Advances in vascular imaging and catheter/guidewire technology, in addition to acquired catheterization skills and a commitment to patient care, place interventional radiologists in a strong position to become an important part of this service. The development of a successful venous access service requires a knowledge of the available devices and their indications, insertion and removal techniques, and the management of complications relating to these devices.
 Access Devices
Access Devices
Modern central venous access devices are available in many sizes and forms. Regardless of these differences, they all result in the positioning of a catheter in the central venous circulation: the superior vena cava (SVC), inferior vena cava (IVC), or right atrium. Early catheters were made of polyethylene and frequently were complicated by thrombosis and infection.6 Current devices are composed of either silicone rubber or polyurethane attached to either an external connector or an implantable port. Silicone is a soft material that has a high coefficient of friction, making catheters composed of this material difficult to use with over-the-guidewire techniques when conventional stainless steel guidewires are used. Newer hydrophilic guidewires can be used with this material. Silicone catheters have been used in the central venous system since the early 1970s and are extremely biocompatible and safe. Polyurethane is a newer material that is stronger than silicone, which allows a catheter to have a larger internal diameter while maintaining the same outer diameter. Polyurethane also can be used with conventional guidewires.
Catheters have either a simple end-hole, a valved tip, or a staggered tip. Conventional end-hole catheters are the most common variety and are available with single, dual, or triple lumen. These catheters can be trimmed at the tip for proper sizing.7 Valved-tip catheters have specially designed slits just proximal to a closed tip, allowing blood to be withdrawn and solutions to be infused but, when not in use, will not allow blood to enter the catheter. The potential advantage of these types of catheters is that they do not require routine heparinization to prevent catheter thrombosis. These devices can only be trimmed at the hub and therefore have a removable external connector.8 Staggered-tip catheters are dual-lumen devices designed for treatments requiring simultaneous rapid aspiration and infusions (pheresis, hemodialysis) with limited admixture. These devices cannot be trimmed from either the tip or hub and therefore are available in multiple lengths to fit the individual patient’s anatomy.
Central venous devices can be separated into peripherally inserted central catheters (PICC), chest-wall external catheters (nontunnelled and tunnelled), and subcutaneous ports (chest wall and extremity) (Figs. 35-1 through 35-3).
PICCs allow external access and are inserted via an upper extremity vein (see Fig. 35-1 and Table 35-1). Because they are inserted peripherally, they carry a lower procedural risk than do centrally inserted catheters and may be preferred by patients who dislike having catheters on their chest.9 In many institutions, PICCs are placed at the bedside by trained nurses; however, for patients with venous occlusions from previous venipunctures and intravenous lines, blind placement of these devices is frequently unsuccessful. Ultrasound or venography often will allow successful placement by the interventional radiologists.9,12
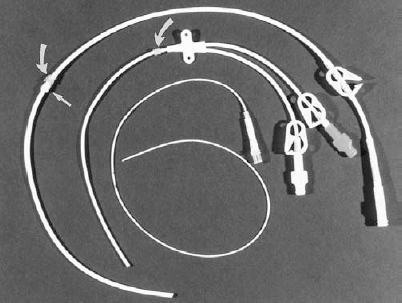
FIGURE 35-1. Photograph of external venous access devices: From top to bottom, single-lumen Hickman catheter, dual-lumen Hohn catheter, single-lumen peripherally inserted central catheter (PICC). Only the Hickman catheter has a Dacron cuff (straight arrow) that is placed in a subcutaneous tunnel. The Hickman and Hohn catheters have a Vita cuff (curved arrows) placed at the entry site
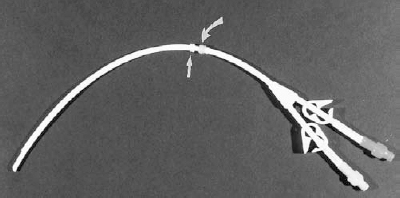
FIGURE 35-2. Photograph of dual-lumen, staggered-tip dialysis catheter.Straightarrow:Dacroncuff.Curved arrow:Vitacuff.
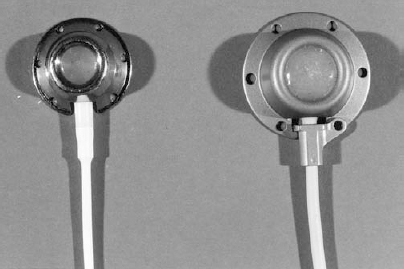
FIGURE 35-3. Photograph of subcutaneous ports: Left: Low profile chest-wall port used in small adults and in children; Right: Standard chest-wall port used in average to large adults.
Chest wall catheters
Nontunnelled
Chest-wall external catheters serve many needs. Acutecare catheters are tapered, nontunnelled catheters that are placed routinely in the units and on the floors for relatively short periods (i.e., days to weeks). In cases where access is difficult (e.g., obesity, coagulopathy, venous thromboses), the interventional radiologist may be asked to place these devices. Nontapered, nontunnelled chest wall external catheters are also available with a single or dual lumen and intended for intermediate-term care (i.e., several weeks to several months) (see Fig. 35-1).13 Because they are nontapered and composed of silicone, they are more difficult to insert than the acutecare catheter and typically are placed near the surgeon or interventional radiologist.
Tunnelled
All tunnelled chest-wall catheters have a small-circumference Dacron cuff attached to the shaft that is positioned within the subcutaneous tunnel. Subcutaneous tunnelling provides mechanical stability and protects against infection from the skin. The Dacron cuff allows ingrowth of fibrous tissue, which secures the catheter within the tract (4–6 weeks) thus creating a long-term device (i.e., months to years).14 Some catheters are also available with a second cuff composed of a silver-impregnated collagen (VitaCuff; Vitaphore, Menlo Park, CA) that is placed at the catheter exit site to serve as an antimicrobial barrier (see Figs. 35-1 and 35-2).
Subcutaneous ports
All implantable devices have a port component buried in the subcutaneous space connected to a catheter with its tip placed in the central venous system (see Fig. 35-3).14,15 The ports are available with single or dual chambers and are constructed of stainless steel, titanium, or plastic [magnetic resonance imaging (MRI) compatible]. The traditional reservoir port is accessed by the use of a noncoring needle that enters the port via a compressed silicone disc. Subcutaneous ports are now available in a wide range of sizes that can better accommodate the patient’s size and amount of subcutaneous tissue (see Fig. 35-3). Small ports are also available for extremity placement in the forearm or upper arm.9,16
 Device Selection
Device Selection
The appropriate choice of a device depends on multiple factors: frequency, length, and type of therapy and use and personal preference (e.g., physician, nurse, home health care personnel, patient). Frequent access (daily) will favor the choice of an external catheter, whereas infrequent use (weekly, monthly) favors a port. Ports are significantly more expensive than external catheters, but they require significantly less maintenance when not in use. Therefore, when the devices are used infrequently, ports become more cost efficient when in place approximately 6 months or longer. Because of the different diameters and thicknesses of the port septum in chest wall (standard size) and extremity ports, the chest-wall ports will accept twice as many needle punctures.
Multiple-lumen catheters have a higher infection rate compared with single-lumen devices. Therefore, the device with the fewest lumens required should be selected. A single-lumen device should be selected for single use or nonsimultaneous multiple uses, whereas a multilumen device will be needed for multiple simultaneous uses. Often, the type of therapy will affect the device choice.4 If blood drawing will be needed, devices larger than 3 or 4 Fr will be helpful. If a triple-lumen device is required, a port or PICC is not a possibility. If high flow rates are required (pheresis, hemodialysis) staggered tip external catheters will be needed.
Finally, the personal preference of the physician may be a factor but should not be the overriding consideration. A device must be selected that will accomplish the treatment plan. Physician preference is the least important factor. The interventional radiologist should be able to place all types of devices. It is much more important to consider the opinions and experience of the health care personnel that will be accessing and managing the device over the long term as well as the feelings of the patient. If a variety of options exist, they should be discussed with the patient before the procedure.
 Placement Techniques
Placement Techniques
Percutaneous placement of long-term central venous access devices requires three basic procedural steps: (a) venous access, (b) formation of a subcutaneous tunnel or pocket, and (c) placement of the catheter into the central venous circulation.
Venous access
Conventional access sites include the subclavian vein (SCV), axillary vein, internal and external jugular veins, and the cephalic and basilic veins of the upper extremity. The choice of access site depends on the device to be placed, venous patency, existing access, and patient preference. Nontunnelled and tunnelled catheters as well as chest wall ports are routinely placed via SCV or axillary access.7,15 The internal jugular (IJV) vein is preferred for the placement of dialysis catheters to avoid injury to the SCV and complications following upper-extremity shunt or fistula placement.17 The right IJV also is preferred to minimize catheter malposition when fluoroscopic guidance is unavailable because of its direct relationship to the SVC and right atrium. PICC are routinely placed through the antecubital veins or the basilic and cephalic veins in the upper arm.10
A routine ultrasound examination of the conventional access sites is performed to determine venous patency. When all conventional access sites are occluded, a variety of unconventional sites are considered, including the inferior vena cava (via translumbar or transhepatic approaches), hepatic vein, collateral channels, and occluded venous segments.18 A patient with an existing catheter usually will have a new site chosen for the device to minimize the risk of infection. In cases of difficult or limited access sites, the existing site may be used. Indwelling tunnelled catheters also can be exchanged for new catheters using conventional guidewire exchange techniques, particularly when the tunnel has matured.
Most patients prefer to have the device placed on the nondominant side if possible. Previous surgery (e.g., mastectomy) or radiation therapy will dictate contralateral access.
Subclavian/axillary vein
Access techniques to the subclavian vein include (a) standard percutaneous insertion using bony landmarks, (b) fluoroscopic guidance with or without venography, and © ultrasound guidance. The standard “blind” technique describes advancing the entry needle along a horizontal plane under the medial two thirds of the clavicle toward the suprasternal notch. Entry into the SCV is quite medial.
Direct puncture of the axillary or SCV can be made under direct fluoroscopic vision during contrast administration via an extremity vein or placement of a guidewire via an antecubital or transfemoral approach (Fig. 35-4).19 When contrast is being used, a preliminary injection is performed to document patency and to select an appropriate skin site. Following preparation, a second contrast injection is performed, and when the vein is opacified, the needle is advanced at an oblique angle (45 degrees) with a tightly collimated field. The vein will be indented initially by the needle and then entered. Safe entry into the SCV also can be accomplished by using the first rib as a fluoroscopic marker.20 A skin nick is made at the lateral margin of the second rib, and the 21-gauge needle is obliquely inserted to hit the anterior lateral first rib immediately caudal to the most lateral extent of that rib. With the most lateral aspect of the first rib 90 degrees from vertical, the SCV will cross the first rib between 85 and 104 degrees in 82% of patients.20 This technique has proved to be an alternative to contrast administration or ultrasonography.
Ultrasound guidance using a 5- or 7.5-MHz linear transducer is our preferred guidance method for venous access (Fig. 35-5). Ultrasound guidance confirms venous patency, allows a more peripheral entry and reduces the risk of pneumothorax and inadvertent arterial puncture.4,21 It is absolutely critical to enter the vein lateral to the first ribclavicle junction to prevent the “pinch-off” syndrome.22,23 When punctures are made more medial, the catheter will traverse the costoclavicular ligament and the tendon of the subclavius muscle. Subsequent arm motion will compress the catheter and lead to fracture and an intravascular foreign body. The SCV/axillary venous segment can be imaged in either the transverse or longitudinal plane (see Fig. 35-5). Longitudinal imaging allows constant identification of the needle tip, whereas transverse imaging allows simultaneous imaging of the vein and adjacent artery.24 Venous entry should be located between the lateral margins of the first and second ribs. The needle is advanced until the vein is indented. A short thrust is then necessary to puncture the wall. Blood should be freely aspirated before guidewire insertion. Contrast confirmation can be performed but is not necessary with ultrasound guidance. The course of the 0.018-inch guidewire should be observed to ensure that it enters the right atrium and not the aorta and left ventricle (indicating an inadvertent arterial puncture). Note that pulsatile blood (signifying arterial entry) will not occur when a 21-gauge needle enters an artery. The needle should not be advanced farther beyond the vein (avoiding a pneumothorax) but partially withdrawn and redirected when venous entry is not successful. Following placement of the mandril guidewire, a transition catheter is placed into the venous system.
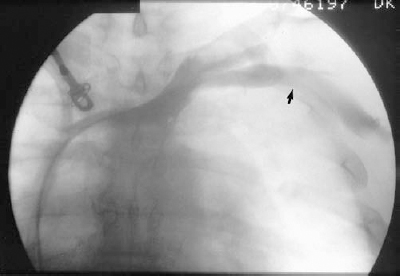
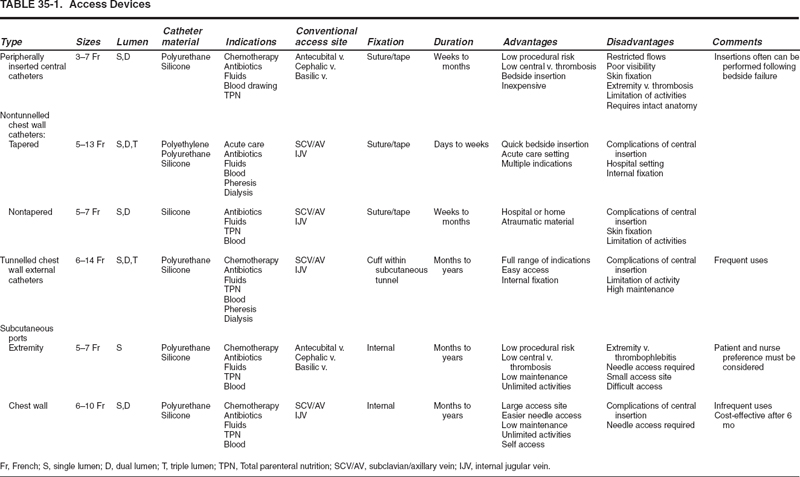
FIGURE 35-4. Venogram of the axillary/subclavian venous segment for venous access. Location where venous entry should be made, lateral to the first rib/clavicle junction (arrow).
Internal jugular vein
IJV access can be accomplished by using standard or ultrasonography-guided techniques. The most common standard approach to the IJV consists of retracting the carotid artery medially while the needle is inserted at a point midway between the angle of the mandible and clavicle directed toward the ipsilateral nipple. The IJV and immediately adjacent carotid artery are easily imaged with ultrasonography. Displayed in the transverse orientation, the IJV and carotid artery will be side by side, or the vein will lie anterior to the artery (Fig. 35-6). With ultrasound guidance, a rather low site is selected between the two heads of the sternocleidomastoid muscle. Ordinarily, this low approach increases the risk of a pneumothorax when standard techniques are used, but this is not a concern with ultrasonography guidance.24 A short (4 cm) 21- or 18-gauge needle is used and directly inserted into the IJC. A short, brisk thrust is needed to enter the vein. The appropriate guidewire is then advanced to the right atrium.
Extremity veins
Access for the radiological placement of PICC and extremity ports is most commonly by the cephalic or basilic veins in the upper arm. These devices also can be placed following access of the antecubital veins. Venous access for the upper arm veins is accomplished by either fluoroscopic guidance during contrast administration or by ultrasound (Fig. 35-7). Entry usually is made halfway between the elbow and axilla. When the fluoroscopic method is used, an intravenous line is started in the antecubital fossa or more distally in the arm.25,26 A venogram is initially performed to identify an appropriate vein to enter. The vein should be of adequate size (at least 3 to 4 mm in diameter) and should lead directly to the central circulation. Following preparation of the upper arm, a contrast injection is repeated to select the specific site of entry. A local anesthetic is placed and a small dermatotome created. During another contrast injection, a 7-cm, 21-gauge needle is inserted into the vein followed by the 0.018-inch guidewire. If venospasm is present, intravenous nitroglycerin should be administered in small aliquots of 100 to 200 μg.
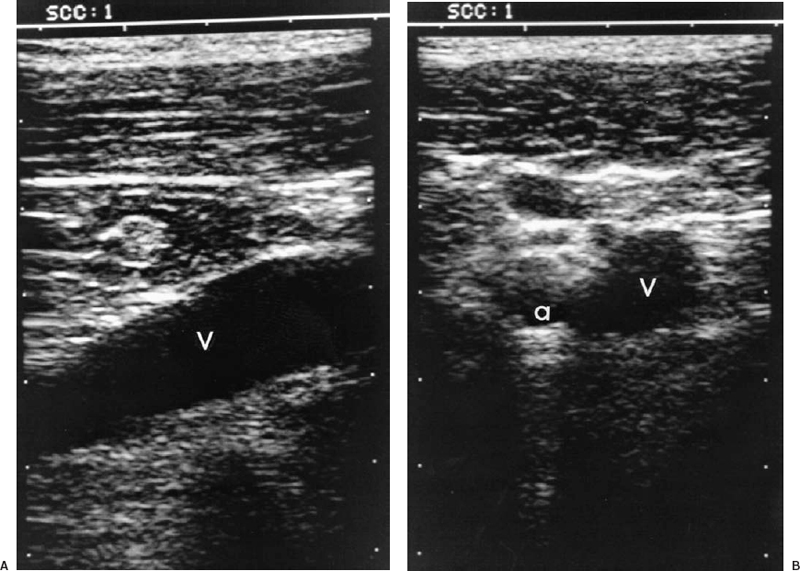
FIGURE 35-5. Subclavian/axillary ultrasound for venous access. A: Longitudinal view of large axillary vein (V). B: Transverse view of axillary artery (a) and vein (V). The vein is located just inferior to the artery in this location. Safe access requires image guidance.
Veins in the upper extremity also can be imaged by using ultrasound.24 The veins are imaged in the transverse plane, and the needle is guided directly into the vein. Following free return of blood, a contrast injection should be performed to confirm a continuous path to the heart. The contrast injection can be performed following the placement of a small (3 Fr) dilator.
Unconventional venous access
When conventional sites are occluded, successful catheter placement still can be accomplished by using unconventional sites. The IVC can be accessed via a translumbar approach similar to translumbar aortography (Fig. 35-8). If the common femoral veins are patent, a guidewire can be easily placed into the IVC to serve as a fluoroscopic marker. The chosen skin site is just superior to the iliac crest to allow a 45-degree medial and slight cephalad angulation. Oblique fluoroscopy is used to direct the 21-gauge diamond-tipped needle to the target. If a guide-wire was not inserted, the needle is directed to the L-3 vertebral body. Once bony contact is made, the needle is withdrawn slightly and redirected anteriorly. The stylet is removed, and aspiration is performed as the needle is withdrawn until blood is returned. The 0.018-inch mandril guidewire then is inserted, followed by a transition dilator.27–31
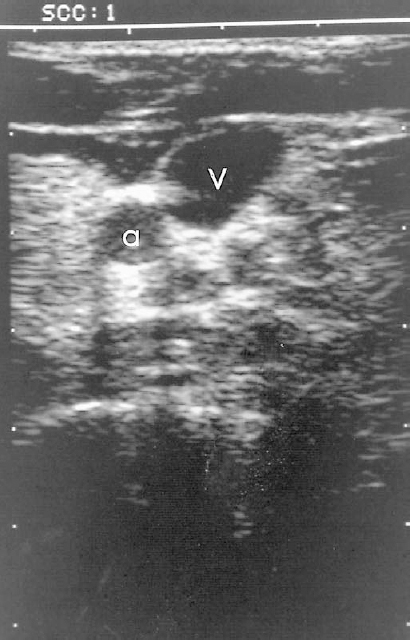
FIGURE 35-6. Transverse ultrasound of the internal jugular vein (V) and carotid artery (a). Access is easily accomplished using transverse imaging.
If the infrarenal IVC is occluded, the suprarenal IVC can be accessed either by a direct transhepatic approach or through a hepatic vein.32 We prefer to access the IVC via a peripheral hepatic vein to maximize intravascular catheter length and to facilitate subsequent manipulation. Hepatic vein access usually is accomplished by either a subcostal or intercostal approach using ultrasound guidance. The middle hepatic vein is most suitable for ultrasound targeting because of its anterior course. The hepatic veins also can be accessed using fluoroscopic guidance in which percutaneous transhepatic cholangiography (PTC)-like passes are made through the liver with a 21- or 22-gauge needle and withdrawn during aspiration. When blood is returned, contrast is injected to confirm hepatic vein entry. Hepatic-vein access has been most useful in children with short-gut syndrome in whom all other sites have become occluded.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree