CERVICAL METASTATIC DISEASE AND THE “UNKNOWN” PRIMARY
KEY POINTS
- Imaging is critical to planning the treatment for cervical metastatic disease.
- The use of imaging to detect the risk of micrometastases based on size criteria is overrated compared to historical risk data.
- No imaging study can eliminate the risk of micrometastases compared to the historical risk profile data in combination with data about the primary tumor in a way that justifies altering traditional decision making based on imaging findings alone.
- The full extent of gross metastatic disease can be known.
- Imaging can find otherwise undetectable head and neck primary cancers.
- Surveillance imaging can modify treatment plans.
INTRODUCTION
Etiology
The vast majority cervical node metastases are from squamous cell carcinoma (SCCA), predominantly from head and neck cancers of mucosal and skin origin. Some cervical metastatic disease, especially when confined to the low neck and supraclavicular area, is from primary sites of origin below the clavicles or due to lymphoma. This discussion is primarily one of the natural history of neck node metastases due to SCCA, but the other patterns of “solid tumor” malignant adenopathy will be considered. This model of that natural history also explains the behavior of metastases from most other tumor types and origins elsewhere in the head and neck. Where natural history varies with different histology and specific primary sites, it is discussed in conjunction with those particular tumor types and sites of origin and in more general terms in Chapters 21 through 24. Primary lymphoreticular processes and their tendencies to show related adenopathy are also discussed as pathologic entities in Chapters 26, 27, 28, and 159. This should be contrasted with the pathophysiologic tendencies of reactive and infectious adenopathy presented in Chapters 13 and 158.
Primary site management in SCCA and other head and neck carcinomas is closely linked to management of cervical lymph node metastases. Failure to control a metastatic tumor in the neck should be an unusual outcome unless the neck disease is very advanced; if the primary is controlled; and surgery, radiation, and chemotherapy have been used optimally. The clear exceptions to a good outcome are advanced neck disease with extranodal spread and fixation to the floor of the neck and/or carotid artery encasement. The specific plan for management of the neck will vary with the primary site treatment plan; therefore, the origin of the neck disease, as well as its extent, becomes pivotal in clinical decision making.
Prevalence and Epidemiology
Factors Modifying the Risk of Lymph Node Metastasis
The likelihood of lymph node metastasis is generally increased in more poorly differentiated tumors, ones that penetrate more deeply at the primary site, and by the capillary lymphatic density at the primary site.1,2 Vascular space invasion at the primary site predicts a high rate of nodal metastasis. Lymphatic spread also increases with recurrent lesions.
All histologic malignancies can show lymphatic spread. The factors just mentioned are probably more important than the specific tumor type with regard to risk of regional disease. For example, adenocarcinoma of the maxillary sinus is less likely to metastasize to nodes than the same histology arising in the parotid gland, assuming the same degree of differentiation because of differences in capillary lymphatic density at these two sites.
The risk of subclinical disease in the lymph nodes in a patient with a clinically negative neck has been understood for many years.3,4 A sample of this classic experience is presented in Figure 157.1. These incidences are discussed in detail in chapters on specific primary sites. In general, the risk of regional disease increases with the T stage. Lymph node involvement to “first echelon” nodes and then contiguous groups is usually predictable based on the primary site.3,5 Skips occur due to variations in capillary lymphatic anatomy. Substantial rerouting of lymphatic spread is caused by prior surgery and/or radiotherapy (RT).6
Midline primary lesions or lateralized primaries that grow to the midline may spread to both sides of the neck. Lateralized lesions will generally spread to ipsilateral nodes unless the primary site is one known for crossed lymphatic drainage. A large nodal mass may obstruct lymphatic vessels and reroute flow. Obstruction of the lymphatic pathways caused by surgery and/or radiation therapy may also shunt the lymphatic flow across the midline mainly through anastomoses in the submental area, as demonstrated in a brilliant and seminal work by Ugo Fisch.6
If a well-lateralized cancer metastasizes to contralateral nodes, level 2 is most commonly involved. A rare contralateral skip to level 3 is possible mainly in tongue cancers due in part to normal crossed drainage of capillary lymphatics.5 Whenever lymph node metastases appear in an unexpected distribution, a second primary must be suspected.
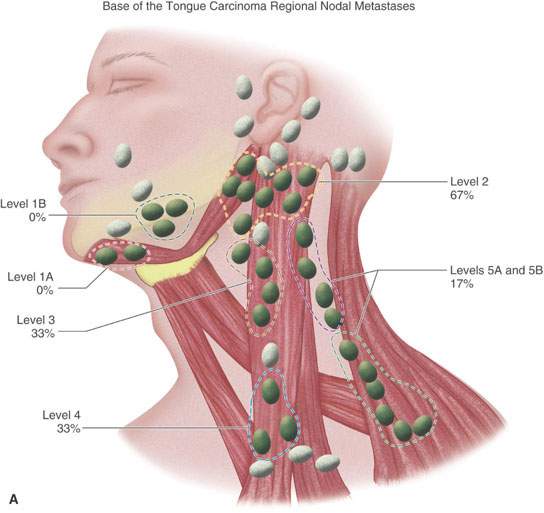
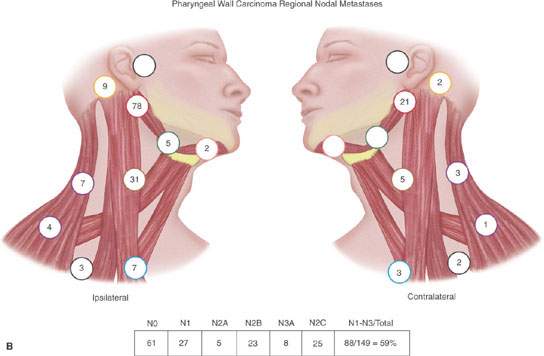
FIGURE 157.1. A, B: Diagrams modified from the work of Lindberg and Byers et al. illustrating that the expected pattern of cervical lymph node metastases from particular head and neck squamous cell primary sites has been well known for about 40 years. Such prior knowledge must be taken into account when there are departures from traditional methods of treatment that have been based on this core knowledge for many years. These patterns help to explain what down side risk might exist in “modernizing” treatment plans. (Part A adapted from Lindberg RD. Distribution of cervical lymph node metastases from squamous cell carcinoma of the upper respiratory and digestive tracts. Cancer. 1972;29:1446–1449 and part B adapted from Byers RM, Wolf PF, Ballantyne AJ. Rationale for elective modified neck dissection. Head Neck Surg. 1988;10:160–167.)
Extracapsular Spread and Other Factors Important to Diagnosis, Prognosis, and Clinical Decision Making
Snow et al.2 reviewed 326 radical neck dissection specimens. Extracapsular spread was documented in 23% of nodes <1 cm in diameter, 53% of nodes 2 to 3 cm, and 74% of nodes >3 cm in that work (Fig. 157.2). Such extracapsular spread has been associated with an approximately 50% decrease in survival in the past. This and other work1,2 has suggested that the number of involved lymph nodes do not correlate with the risk of extracapsular spread.
Lower neck regional disease is likely associated with an increased incidence of distant metastasis. Extracapsular spread, many positive nodes, and low neck disease in aggregate might, therefore, constitute a profile that justifies the use of fluorine-18 2-fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) to look for distant disease.
Clinical Presentation
General
Metastatic disease most frequently is discovered as a neck mass, either as the presenting sign of a cancer or, more commonly, by physical examination and/or imaging studies done to evaluate a known head and neck region primary cancer. If the origin of the neck mass is uncertain after initial imaging, fine needle aspiration (FNA) biopsy of the palpable mass frequently is the next step to confirm that it is a nodal mass due to cancer.
The neck examination can be a very reliable source of clinical evaluation if learned correctly. However, even a well-trained examiner sometimes has difficulty identifying or excluding disease in the neck. This is especially true in the posttreatment neck. Imaging is particularly useful in a patient with a short, thick, and/or obese neck and the posttreatment neck. Once a metastatic node is suspected or confirmed, imaging is indispensable for assessing nodes not accessible on physical examination, such as retropharyngeal nodes, highest level 2 (retrostyloid space) nodes, and the deeper level 6 nodes (Fig. 157.3).
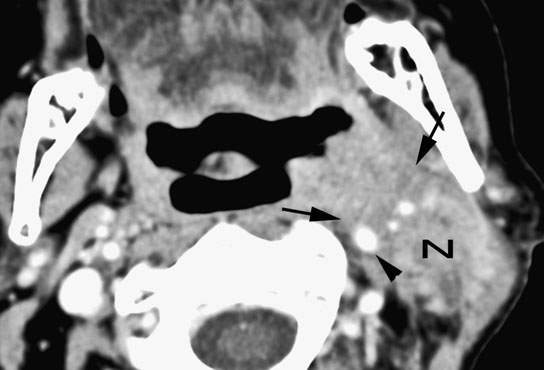
FIGURE 157.2. Primary tonsillar cancer with aggressive infiltrating margins (arrows) leading to part of the involvement of the carotid artery. The level 2B metastatic node (N) shows equally aggressive margins with extranodal spread likely fixed to the deep neck and sternocleidomastoid muscle and contributing to the internal carotid involvement (arrowhead). (NOTE: With this type of imaging data, a better strategy can be adopted than the initially planned gross total resection of the tumor, which would have resulted in gross residual tumor being left behind.)
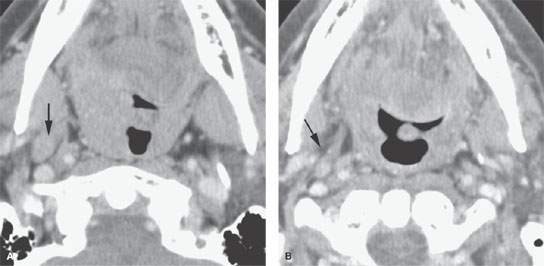
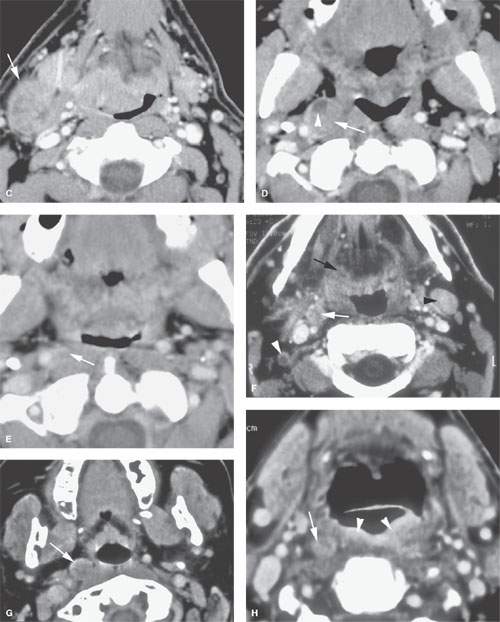
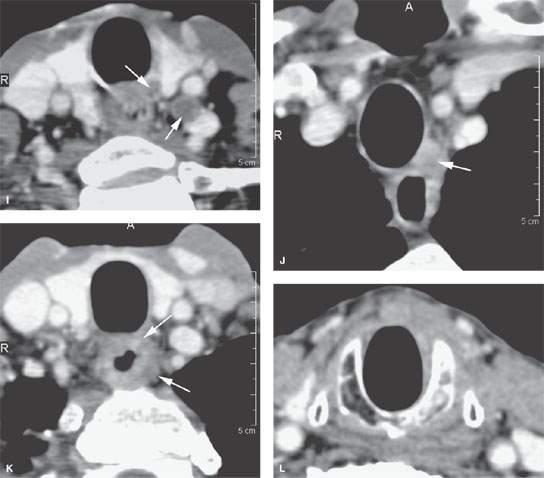
FIGURE 157.3. (Continued) F: Patient 3 with an anterior tonsillar pillar carcinoma that extended into the glossotonsillar sulcus (black arrow). The patient elected surgical treatment. The positive parapharyngeal node (white arrow) was removed at surgery. An additional level 2B node was confirmed as positive (white arrowhead). The left level 2A node was reactive (black arrowhead). G: Patient 4 with a retromolar trigone carcinoma. This study shows a positive retropharyngeal lymph node (arrow). The patient was treated with primary surgical resection. The retropharyngeal lymph node was removed by blunt dissection, and metastases was confirmed. Disease was confined to the lymph node. The patient survived. H: Patient 5. A posterior pharyngeal wall carcinoma (arrowheads) and a related retropharyngeal lymph node at the level of the hyoid bone (arrow). (NOTE: Before the advent of CT, patients with posterior pharyngeal wall carcinoma had a significant failure rate likely due to underradiation of positive retropharyngeal lymph nodes in an effort to keep the dose to the cervical spinal cord acceptable. Modern radiotherapy techniques allowed for adjustment to treat these relatively posteriorly placed nodes, which improved the rate of control of that disease.) Contrast-enhanced computed tomography (CT) images of seven patients with parapharyngeal and retropharyngeal lymph node metastases that have the potential to alter treatment plans. A, B: Patient 1 showing a clinically unexpected high level 2 or retrostyloid metastatic lymph node (arrow in A) that responded completely to radiotherapy (arrow in B). C–E: Patient 2 with a tongue base primary tumor as seen in (C) and related level 2 adenopathy with evidence of early capsular penetration (arrow). In (D), there is a positive right retropharyngeal lymph node with both a solid (arrow) and more peripheral cystic component. In (E), the retropharyngeal lymph node is controlled by radiotherapy alone (arrow). (NOTE: Retropharyngeal adenopathy is known to occur with predictable frequency up to about 10% to 15% in patients with primary carcinomas of the tonsil and tongue base. These nodes are much more commonly involved with nasopharyngeal carcinoma. There is also an incidence of their involvement in pyriform sinus and posterior pharyngeal wall carcinoma.) I–K: Patient 6 with a presenting complaint of fullness in the left neck and mild dysphagia. CT showed level 6 adenopathy both in the tracheoesophageal groove and medial to the carotid sheath (arrows in I). Left paratracheal adenopathy was present in the tracheoesophageal groove (J), and the infiltrating primary tumor was identified by imaging. Imaging findings directed the endoscopy to the site that yielded confirmation of cervical esophageal carcinoma. L: Patient 7 with transglottic carcinoma of the larynx demonstrating a positive prelaryngeal level 6 lymph node (arrow) associated with spread of tumor through the cricothyroid membrane and likely cartilage invasion based on the sclerosis of the left cricoid ring.
Normal structures that may be confused with a positive level 2 lymph node include transverse process of C1 or C2; the tail of the parotid gland; and a prominent, calcified carotid bifurcation. In level 1, it may be impossible to distinguish abnormal nodes from an abnormal submandibular gland. These normal sources of potential confusion are typically sorted out with imaging.
The Unknown Primary
Primary SCCA of the pharynx often presents with metastatic cancer in neck lymph nodes (Fig. 157.4). The primary is easily identified by physical examination in about 97% to 98% of patients.7 About 2% to 5% of the time, the primary cannot be found even by complete endoscopy by an experienced examiner.
Spontaneous remission of the primary may occur since some patients treated solely by neck dissection are cured without the primary site of origin established even by long-term follow-up. Spontaneous regression of other primary tumors has been reported. The concept of a cancer arising in branchial cleft remnants of the lateral neck should be abandoned even though it may rarely be the explanation (Figs. 153.18 and 157.4). Assuming a branchial origin can impede the search for a primary and lead to inadequate treatment and follow-up.
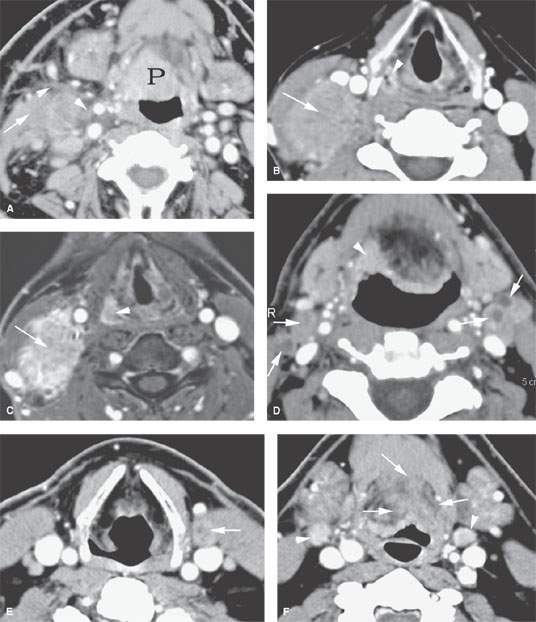
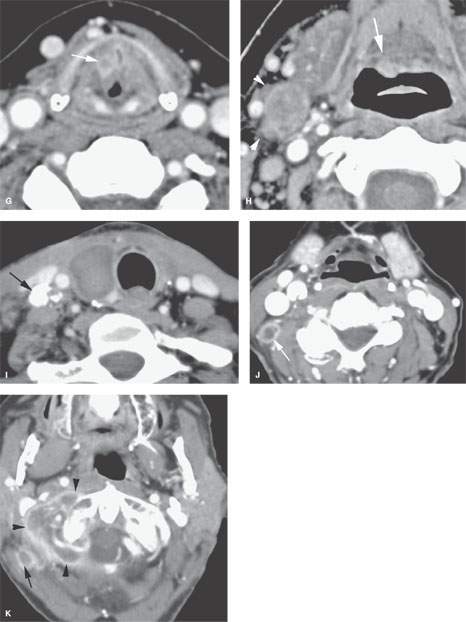
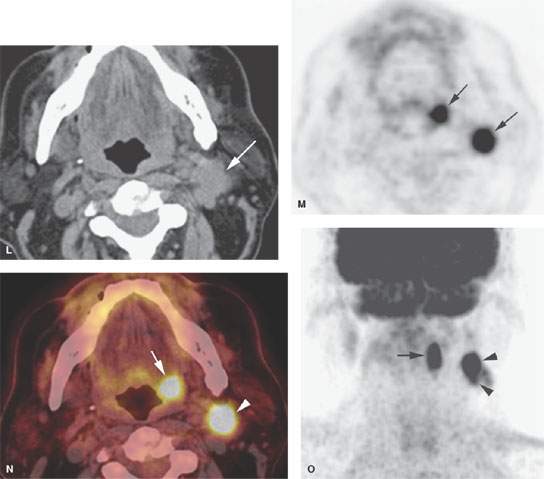
FIGURE 157.4. A series of patients presenting with a neck mass and physical examinations by very experienced providers showing no evidence of a primary tumor. A: Contrast-enhanced computed tomography (CT) in Patient 1, showing an aggressive infiltrating level 2 mass infiltrating the sternocleidomastoid muscle (arrow) and extending out of the capsule and into the perinodal soft tissues (arrowhead). The responsible primary tumor at the tongue base was discovered by imaging and confirmed by biopsy. B, C: Patient 2 with dysphagia and right neck mass. There was no tumor visible at endoscopy. Contrast-enhanced CT shows the aggressive lymphadenopathy in the right neck (arrow) and the likely primary tumor in the right pyriform sinus (arrowhead). In (C), the T1-weighted fat-suppressed magnetic resonance image confirms the aggressive right neck lymphadenopathy invading the sternocleidomastoid muscle and possibly adherent to the common carotid artery (arrow). The primary tumor was again identified in the pyriform sinus, and directed biopsies in that region confirmed the small primary tumor responsible for the lymphadenopathy. D: Patient 3 presenting with otalgia and bilateral cervical adenopathy. Multiple positive lymph nodes are present in level 2A on both sides (arrows). The primary tumor was found by this anatomic imaging with contrast-enhanced CT (arrowhead), and directed biopsy returned squamous cell carcinoma (SCCA). E, F: Patient 4 with a neck mass on the left in level 3. Contrast-enhanced CT confirmed metastatic adenopathy (arrow) as seen in (E). The primary tumor infiltrating the tongue base is shown by the arrows in (F). Diffusely enhancing lymph nodes in level 2A are also present. Their tumor status was never proved since the patient responded completely to chemoradiation without neck dissection. G, H: Patient 5 with laryngeal carcinoma shown by the arrow in (G). The patient also had a level 2A palpable lymph node (arrowheads in H). Isolated level 2 lymphadenopathy is very unusual in a laryngeal primary; thus, this node prompted a search for a second primary, and a small predominately exophytic tumor was suspected on the basis of this examination and confirmed by biopsy (arrow). I: Patient 6 with a right lower neck mass of uncertain etiology. CT revealed partially calcified and partially cystic enhancing lymph nodes in the right lower neck (arrows) secondary to thyroid carcinoma. J, K: Patient 7 presenting with a right mid to upper posterior triangle neck mass believed to be adenopathy. CT confirmed level 3 adenopathy (arrow) in (J). In (K), another node in the posterior triangle is shown, with the source being metastases from a primary lung cancer metastatic to the C1 lateral mass (arrowheads). (NOTE: Patient 7 is an example of how imaging can significantly alter the diagnostic approach in cases of neck masses of uncertain etiology.) L–O: Fluorine-18 2-fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) may be used to find a clinically inapparent primary tumor. A non–contrasted-enhanced axial CT image (L) at the level of the mandible reveals an enlarged level 2A lymph node on the left side (arrow in L). No primary tumor was apparent on clinical examination or on this image. The positron emission tomography (PET) image in (M) shows two focal hypermetabolic areas (arrows) that localize on the fused PET-CT image to the abnormal lymph node (arrowhead in N) and to the tonsillar fossa (arrow in N) on the left side. This was biopsy-proven SCCA of the left tonsil. The coronal PET image illustrates the craniocaudal extent of the primary tonsillar tumor (arrow in O) and of the metastatic lymph node (arrowheads in O).
PATHOPHYSIOLOGY
Anatomy
Capillary Lymphatics
Capillary lymphatic distribution is the major explanation for the patterns and incidence of metastases to regional lymph nodes in head and neck cancer that will be presented in conjunction with those primary sites. The lymphatic metastatic process begins when cancer cells penetrate the lamina propria of the involved epithelial surface to reach the deeper capillary lymphatics.5 There are a few capillary lymphatics in the periosteum and perichondrium and none in bone and cartilage. There are no capillary lymphatics in the globe and few in the orbit. Muscle and fat also have few capillary lymphatics. This explains the very low rate of lymphatic node metastases from tumors arising in these structures and tissues.
Once tumors have penetrated deep enough in a capillary lymphatic–rich area, the risk of lymph node metastases will be related to the depth of cancer invasion.8,9 This trend has been proven and is used in clinical decision making for malignant melanoma of the skin and SCCA of the oral tongue. The nasopharynx and pyriform sinus have the most capillary lymphatic density.5 The paranasal sinuses, middle ear, and vocal cord have few, if any, capillary lymphatic channels.5 This capillary density distribution correlates very well with the high rates of nodal metastases in the pharynx as well as with the near zero rates of lymph node metastases in tumors confined to the paranasal sinuses and true vocal cord.
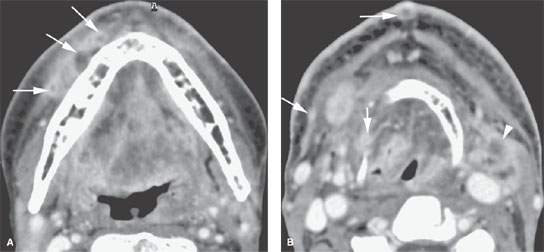
FIGURE 157.5. A patient treated with surgery and radiotherapy for head and neck carcinoma. The patient had increasing edema and neck pain. A: Contrast-enhanced computed tomography showing multiple nodules spreading beneath the superficial musculoaponeurotic system (arrows) as an expression of diffuse lymphatic involvement with cancer. B: In addition to showing the extensive lymphedema throughout the upper neck, imaging showed multiple enhancing nodules in the skin and elsewhere (arrows) consistent with lymphangitic spread of tumor in addition to the obvious recurrent nodal mass at level 2A on the left (arrowhead).
Lymphatic Trunks
Lymphatic collecting trunks form from the capillary lymphatics. Some of the lymphatic vessels have valves, and some do not. This sometimes has grave prognostic significance. In the deeper layers of the skin, the valves direct lymphatic flow in a predictable manner. However, superficial skin lymphatics are valveless, thus a cancer spread may be helter-skelter, resulting in unpredictable remote spread and satellite lesions (Fig. 157.5).
Lymphatic vessels draining the mucous membranes have valves, making their drainage patterns predictable in the previously untreated patient based on the known anatomic range of variation in the vessels.5 The lymphatic trunks empty into a large vein, near the junction of the jugular and subclavian veins at the surgical venous angle (Fig. 149.11). Lymphatic vessels may drain directly into the internal jugular or subclavian veins. There are also direct lymphatic to venous connections between lymph vessels and veins within the lymph node. The lymphatic and venous connections in nodes likely open up mainly after occlusion or obstruction of a lymphatic trunk. The direction of lymphatic flow with known common variations and less common aberrant pathways are well established.5 Variations that affect treatment decisions will be discussed for specific primary sites. Obstruction of lymphatic flow can be caused by tumor, postradiation fibrosis, surgery, or other traumatic interruption.6 The more complete the obstruction, the greater the change in lymph flow and resulting change in the expected pathway and pattern of lymph node metastasis. Postradiation alterations in redirected lymph flow are to some extent RT dose dependent (Figs. 157.5 and 157.6).
Lymph Nodes
About 150 to 350 lymph nodes lie above the clavicles.5 This is about one third of the lymph nodes in the body. This text will use the current standardized numbering systems for the cervical lymph node groups (Fig. 149.10). This system, agreed upon by surgeons, radiation oncologists, and diagnostic radiologists, has eliminated the previously confusing and less precise naming and renaming of these groups. Other important named groups include the retropharyngeal, facial, parotid, mastoid, suboccipital, and posterior neck nodes.
Inconstant, 1- to 2-mm lymph nodules and aggregates called intercalated nodes may lie along the course of a lymph vessel between its origin and its entrance to a larger node. Intercalated nodules and nodes can mimic satellite tumor nodules when involved with metastatic lymphatic spread.
Nodal Groups
One must be thoroughly familiar with the anatomic boundaries of the cervical lymph nodes as currently classified in levels 1 through 6 as well as the retropharyngeal, parotid, facial, lingual, mastoid, and posterior neck groups in order to properly assess metastatic disease from head and neck and other primary sites. The detailed anatomy, including anatomic variants, is discussed in conjunction with the normal anatomy of the neck (Chapter 149). The primary drainage fields of these nodes must also be understood. These include the following:
 Level 1A: The lip, skin of the chin, more anterior buccal mucosa, gingiva, floor of the mouth, and tongue drain to these nodes. The efferent vessels drain to levels 1B and 2A.
Level 1A: The lip, skin of the chin, more anterior buccal mucosa, gingiva, floor of the mouth, and tongue drain to these nodes. The efferent vessels drain to levels 1B and 2A.
 Level 1B: The lips, floor of the mouth, buccal mucosa, gingiva, nasal vestibule, skin of the anterior face, oral tongue, palate, and submandibular and sublingual salivary glands drain to these nodes. The efferent vessels drain mainly to level 2A.
Level 1B: The lips, floor of the mouth, buccal mucosa, gingiva, nasal vestibule, skin of the anterior face, oral tongue, palate, and submandibular and sublingual salivary glands drain to these nodes. The efferent vessels drain mainly to level 2A.
 Level 2: The entire pharynx and larynx and other neck viscera drain to this group of nodes. Level 2 receives connections from levels 1 and 5; thus, they are a final common pathway for much lymphatic drainage.
Level 2: The entire pharynx and larynx and other neck viscera drain to this group of nodes. Level 2 receives connections from levels 1 and 5; thus, they are a final common pathway for much lymphatic drainage.
 Levels 3 and 4: The visceral compartment, mainly the larynx, hypopharynx, thyroid, and cervical esophagus, drain to these nodes.
Levels 3 and 4: The visceral compartment, mainly the larynx, hypopharynx, thyroid, and cervical esophagus, drain to these nodes.
 Level 5: These nodes drain predominantly to the pharynx and larynx. However, they have important connections to parotid nodes and skin lymphatics, which make them very important in the treatment of skin cancers.
Level 5: These nodes drain predominantly to the pharynx and larynx. However, they have important connections to parotid nodes and skin lymphatics, which make them very important in the treatment of skin cancers.
 Level 6: These nodes drain their adjacent viscera and are contiguous with mediastinal lymphatics.
Level 6: These nodes drain their adjacent viscera and are contiguous with mediastinal lymphatics.
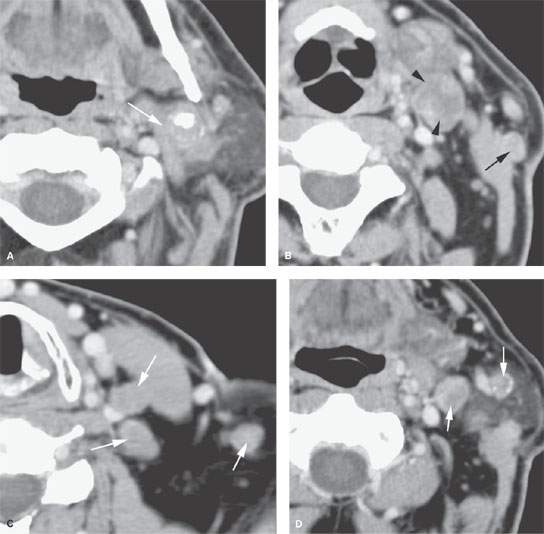
FIGURE 157.6. A patient with oropharyngeal cancer and metastatic adenopathy to level 2 on contrast-enhanced computed tomography. A: A very aggressive level 2 lymph node mass fixed to the posterior belly of the digastric muscle (arrow). B: Fairly typical level 2B metastases. The node is largely replaced by tumor (arrowheads). However, there is the unusual feature of a positive node in the external jugular chain—a finding consistent with lymphatic obstruction and redirected lymph flow. C: Multiple positive nodes are present at levels 3 and 5 (arrows). D: Backup adenopathy into the parotid and external jugular lymph nodes (arrows). (NOTE: This patient illustrates that level 2 adenopathy due to a pharyngeal primary can result in the rerouting of lymphatic flow into parotid nodes. This issue of rerouted lymphatic flow points out the danger of modifying radiotherapy plans to exclude parotid lymph nodes when there is substantial level 2 adenopathy. In the era of intensity-modulated radiotherapy, this has produced failures in parotid nodes when treatment plans have been modified to spare parotid tissue before adequately considering the lessons taught by Fisch and others so many years ago.)
Retropharyngeal nodes drain the nasal cavity, nasopharynx, pharyngeal walls, pyriform sinus, hard and soft palate, middle ear, and eustachian tube.
Parotid nodes drain the skin of the frontal scalp, temple, nose, eyelids, pinna, external auditory canal, and malar area. They also drain the eustachian tube.
Mastoid or retroauricular nodes, occipital nodes, and posterior neck nodes drain the scalp and posterior periauricular soft tissues of the ear.
Pathophysiology and Patterns of Disease
The basic pathophysiology and related pathologic anatomic correlates of cervical lymph node metastatic disease as seen on imaging is due to tumor emboli that are transported by lymphatic channels to become trapped in the subcapsular sinus of a lymph node where the deposits may grow. This focal metastasis can then itself further embolize to a contiguous lymph node. The nodes are prepared in advance by the primary tumor to be areas both receptive to the deposits and are induced to prepare an environment that is favorable to metastatic tumor growth.
The deposits grow—at first possibly mainly peripherally because the afferent capillary lymphatics enter the capsule of the node—within the lymph node, and the metastatic deposits gradually replace its normal architecture (Fig. 157.7). Alternatively, the SCCA cells, especially if they lose the adhesive tendencies more prevalent in the better differentiated epidermoid cancers, might percolate through the lymphatic sinusoids, producing broad zones of altered architecture up to totally replacing the normal nodal architecture (Fig. 157.7J).
Eventually, areas of necrosis develop. The node may become completely replaced by tumor and become largely necrotic and cystic. This gross necrosis is often a late finding and the one most emphasized in the imaging literature as a criterion for metastases. With modern imaging, the metastatic deposits can be recognized much earlier in their natural history as smaller focal defects often as peripheral focal defects in the node (Fig. 157.7). Tumor cells within a node can also enter the venous circulation, possibly leading to pulmonary metastases since the lung capillary bed is a first-order filter of the cervical venous drainage.
The enlarging focal metastasis will continue this local growth and eventually invade the capsule of the lymph node and spread to perinodal tissue (Fig. 157.8). Extracapsular spread may be seen both in lymph nodes only focally involved as well as nodes nearly totally replaced by tumor (Figs. 157.7J and 157.8). Continued extracapsular growth may invade and become fixed to and/or encase surrounding structures.
The risk of cervical lymph node metastases is in large part dependent on the density of the capillary lymphatics at any given primary site. Tumors with less invasive margins are less likely to develop lymphatic metastases. For instance, verrucous carcinoma has indolent tumor margins and almost never invades the lymphatic system. The depth of invasion of the primary cancer also correlates with the risk of lymph node metastasis in mucosal cancer, as it does with malignant melanoma. For instance, in oral tongue cancers, the risk of lymph node metastasis increases rapidly with primary depth of invasion of 2 to 4 mm.8–11 Sarcomas usually originate in tissues with few or no lymphatics, such as muscle, bone, or cartilage, and generally have relatively low rates of lymph node involvement. These and other cancers normally at low risk for nodal metastases may spread by way of the lymphatic system once they involve areas with higher capillary lymphatic density.
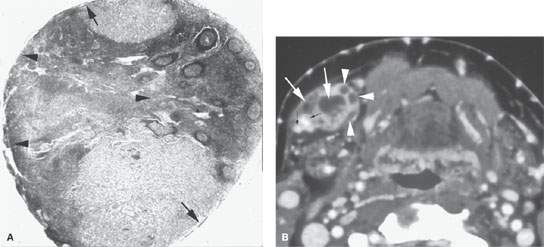
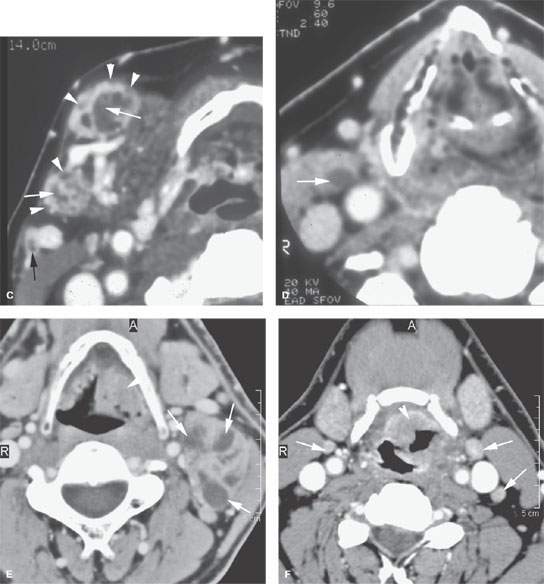
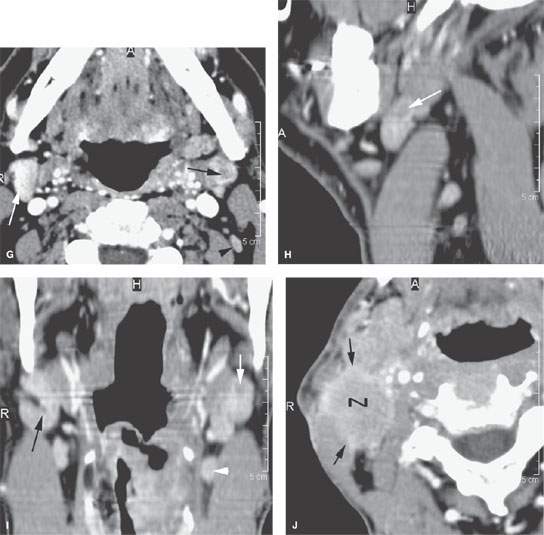
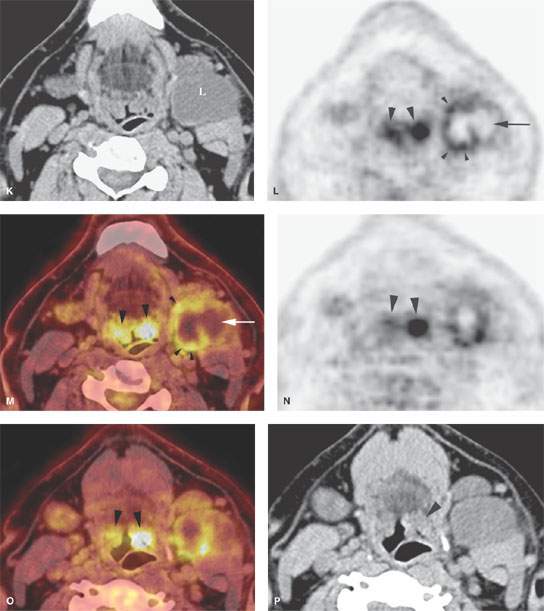
FIGURE 157.7. A: Pathologic specimen of a node to demonstrate the gross morphology of metastatic deposits. Two deposits are seen peripherally in the lymph node respecting the margins of the lymph node capsule (arrow). There is another zone of tumor extending from the capsule along its peripheral margins and more centrally showing cellular necrosis (arrowheads). The remainder of the node parenchyma is relatively normal with lymph node follicles present. Examples of metastatic squamous cell carcinoma manifesting as focal nodal changes in six patients follow. B, C: Patient 1 with a contrast-enhanced computed tomography (CT) study showing metastases to level 1B lymph nodes. The nodes in (B) show focal peripheral (arrowheads) and/or central and larger extending deposits (large white arrows) as well as zones of focally increased density and increased enhancement (small black arrows). In (C), numerous small peripheral deposits are present (arrowheads) respecting the lymph node capsule margin or showing very early capsular rupture. There are larger central deposits of metastatic disease (white arrows). Also note the external jugular node of approximately 4 mm that contains a focus of metastatic disease (black arrow). D: Patient 2 with a clinically negative neck contrast-enhanced CT showing a metastatic deposit (arrow) replacing part of the node parenchyma. E: Patient 3 with a level 2 neck mass of uncertain etiology. Contrast-enhanced CT shows multiple peripheral deposits of metastatic disease in an obviously enlarged node (arrows) and the responsible primary tumor in the vallecula (arrowhead). Despite extensive metastatic disease in the node, there was no capsular penetration. F–I: Patient 4 with a contrast-enhanced CT of known supraglottic carcinoma. In (F), there are lymph nodes present that are within normal limits and size but are enhanced to a generally greater degree than normal (arrows). Such enhancement can be due to reactive changes. The primary tumor was seen in the vallecula. In (G), a section somewhat more cephalad shows a focal rim-enhancing peripheral deposit suspicious for metastatic disease in the left level 2A node (black arrow) and the slightly enlarged diffusely enhancing node in right level 2A (white arrow). The latter is more likely a reactive than a metastatic node. In (H) and (I), sagittal and coronal reformations, respectively, show an obvious metastatic deposit in the left level 2A node (arrows) and the homogeneously enhancing level 3 node (I) seen previously (arrowhead). The enlarged reactive right level 2 node can be seen folded on itself with a central fatty area (black arrow in I). J: Contrast-enhanced CT in Patient 5 with a relatively poorly differentiated squamous cell carcinoma of the tongue base. The level 2A node is grossly enlarged (N). It is slightly inhomogeneous due to it being diffusely infiltrated with metastatic cells and reactive lymphocytes. The node capsule shows slight irregularity, which is diagnostic of early extranodal spread. K–P: Patient 6 with fluorine-18 2-fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) activity in necrotic nodes that may only be confined to areas of remaining viable tumor. A non– contrast-enhanced axial CT (K) from an FDG-PET–CT examination demonstrates a markedly enlarged group 2A lymph node (L, K) on the left side. The axial positron emission tomography (PET) image performed at the same level (L) shows only marked and partially nodular-appearing fluorine-18 2-fluoro-2-deoxy-D-glucose (FDG) uptake (small arrowheads in L) that corresponds to the medial rind (small arrowheads in M) of the enlarged lymph node, as best seen on the fused PET-CT image (M). The majority of the enlarged lymph node does not take any FDG uptake (arrow in L and M). The PET (L) and the fused PET-CT (M) images show asymmetric uptake in the tongue base (large arrowheads in L and M), which is more apparent on the more inferior PET (large arrowheads in N) and fused PET-CT (large arrowheads in O) images. This is suggestive of a possible primary tumor in the left tongue base that was confirmed with biopsy. Although the non–contrast-enhanced axial CT image at the lower level (O) shows hypertrophy of the lymphoid tissue on the left side (large arrowhead in O), there are no signs of deep infiltration on either CT image (K or O). (Part A from Haagensen, Feind, et al., eds. The Lymphatic in Cancer. Philadelphia, WB Saunders 1972, with permission.)
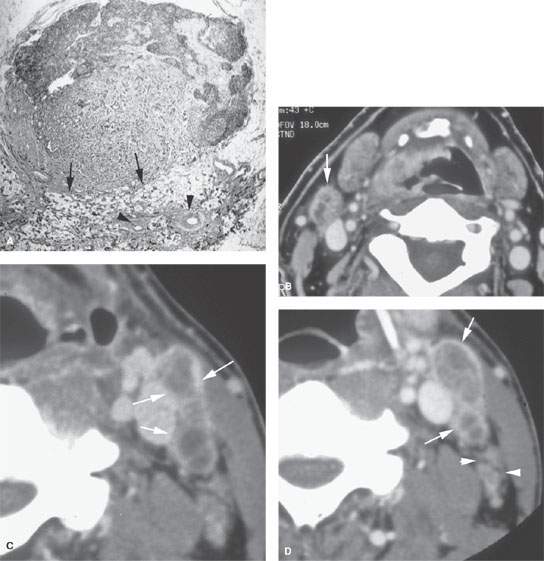
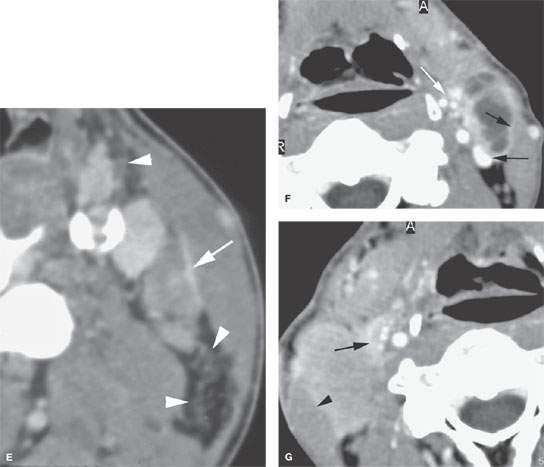
FIGURE 157.8. A: Pathologic specimen that demonstrates a cervical lymph node with metastatic squamous cell carcinoma (SCCA). The metastatic deposit extends beyond the capsule of the node (black arrow) to incite a vascular and fibrous response in the perinodal soft tissue (arrowheads). This is the gross morphology represented in the following computed tomography (CT) studies of four patients with various degrees of relatively early extranodal spread. B: Patient 1 with a level 2A lymph node showing early extranodal spread (arrow) as irregularity at the nodal margin extending minimally into the perinodal fat. C–E: Patient 2 with left neck adenopathy due to metastatic SCCA from an oropharyngeal primary. In (C), the largely necrotic level 3 nodes show capsular enhancement (arrows) with a broad zone of this reactive change along the jugular vein and sternocleidomastoid muscle as evidence of likely extranodal spread and possible adherence to these structures. In (D), the capsular enhancement is fairly well circumscribed—a finding that suggests the tumor may still be confined within the node capsule (arrows). Multiple small positive nodes are present with perinodal changes suggestive of lymphatic obstructive and dilated capillary lymphatics (arrowheads). In (E), there is continued enhancement of the node capsule along the lining of the sternocleidomastoid muscle, which is suggestive of early capsular spread and adherence to the muscle (arrow). Serpiginous areas of abnormality within the anterior and posterior triangle fat (arrowheads) strongly suggest diffuse lymphatic obstruction and dilated capillary lymphatic channels. All of these findings are consistent with early extranodal spread and very likely lymphatic obstruction secondary to metastatic disease. F: Patient 3 with contrast-enhanced CT showing indistinct enhancement of the lymph node capsule along the border of the sternocleidomastoid muscle and in contact with the jugular vein, strongly suggesting early extranodal spread and possible adherence to those structures (black arrows). Tissue planes along the external jugular vein branches (white arrow) suggest extranodal spread involving those vessels. G: Patient 4 with a contrast-enhanced study showing a level 2A node completely replaced by tumor with no well-defined capsule and evidence of early capsular penetration and adherence to the sternocleidomastoid muscle (arrowhead). Tumor also appears to extend outside the capsule of the node, probably invading the jugular vein on the right side (arrow). (Part A from Haagensen, Feind, et al., eds. The Lymphatic in Cancer. Philadelphia, WB Saunders 1972, with permission.)
Imaging of the Normal Lymph Node Size and Morphology: The Baseline
A node’s shape on axial sections depends on its orientation to the transverse plane of the body. This should be taken into account, as largest short axis dimensions are typically used even though they are relatively useless as a criterion for metastatic disease (Figs. 149.7–149.10).
Variations due to intra- and perinodal fatty foci and nodal folding should not be mistaken for focal defects produced by metastases (Figs. 149.7 and 149.8).
Computed tomography (CT) and magnetic resonance imaging (MRI) performed with and without intravenous contrast injection show normal nodes as homogeneous internally. Parenchymal changes seen within nodes on contrast-enhanced magnetic resonance (CEMR) and contrast-enhanced computed tomography (CECT) are very important in recognizing early metastases in nodes. Such changes can be seen with CECT in nodes with maximum short axis measurements as small as 3 to 5 mm (Fig. 157.7). Contrast is also required on CT to distinguish nodes from vessels. At ultrasound examination, normal nodes have a definable interface with surrounding tissues and homogeneous texture (Fig. 4.6). The texture may be interrupted by hilar structures including small nodal vessels; these normal structures can also be seen on good-quality CECT and CEMR studies. Blood flow patterns in the nodal vessels can be studied with color flow Doppler techniques (Figs. 4.1, 4.6, and 4.7 and Chapter 4). Resistive indices can also be calculated. Both resistive indices and flow patterns differ in normal and pathologic nodes. These gross flow features are a reflection of abnormal morphology seen on CT, magnetic resonance (MR), and ultrasound and are also related to perfusion data available on CT and MR studies.
The nodes of the head and neck vary in size as seen on axial CT and MR, normally from 1 to 15 mm in maximum short axis dimension12 (Table 149.2). With ultrasound, and on coronal and sagittal images, the long axis is seen and nodes are often 15 to 20 mm or more (especially in children) in length. Size criteria are very limited with regard to predicting the risk of lymph node micrometastases in otherwise normal nodes, thus considerably limiting the value of size criteria in clinical decision making to almost zero.
Imaging Criteria of Metastatic Disease in Lymph Nodes
For anatomic imaging studies, the diagnosis of metastatic SCCA in lymph nodes depends on size and the presence of focal parenchymal defects, including focal areas of low density or signal intensity, focal enhancement and foci of impacted keratin debris, and/or dystrophic calcification (Figs. 157.3–157.7). Irregularity of node margins, rim enhancement, and more obvious extranodal infiltration of bordering soft tissues help to identify tumor penetrating the node capsule (Figs. 157.2, 157.4, 157.5, 157.8, and 157.9). No criterion is specific for metastatic cancer. Lack of specificity is usually unimportant since the patients have known cancer. The lack of specificity is more of a problem in patients with a neck mass of uncertain etiology.
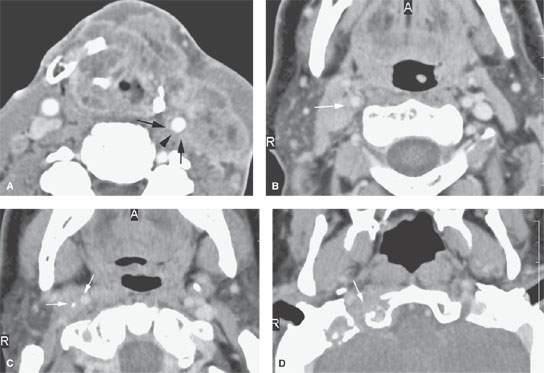
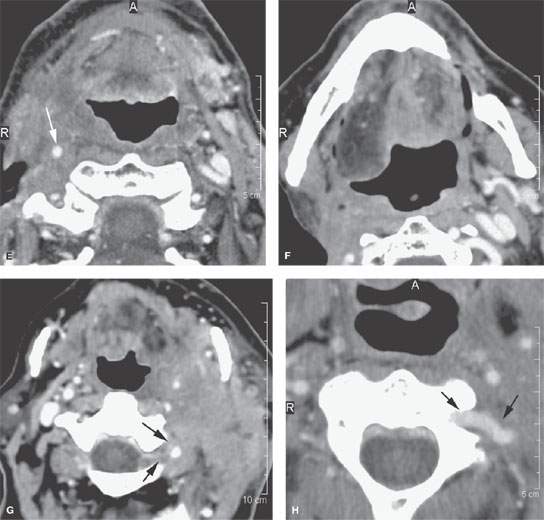
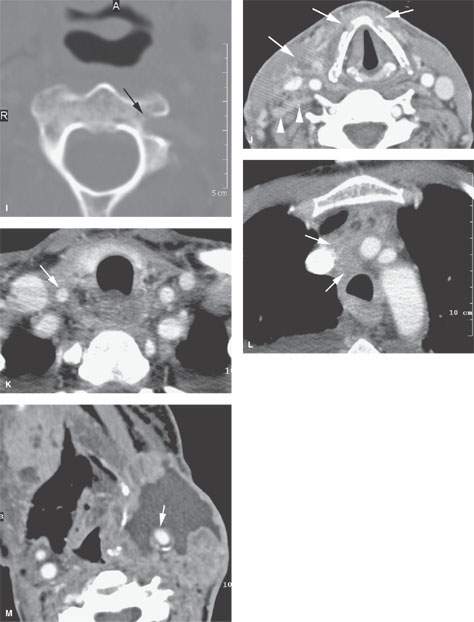
FIGURE 157.9. Several patients illustrating various degrees of advanced extranodal spread and fixation and encasement of structures within the neck. A: Patient 1 with a contrast-enhanced study of an advanced laryngeal carcinoma and related level 3 adenopathy. There is obvious extranodal spread of tumor that surrounds the common carotid artery (arrows), leaving a small gap (arrowhead) where the artery is not completely surrounded. The degree of circumferential involvement strongly suggests carotid encasement. B–D: Patient 2 with carcinoma of the skin. Contrast-enhanced computed tomography (CT) study shows metastatic adenopathy involving retrostyloid high level 2 lymph nodes with extranodal spread encasing the cervical segment of the internal carotid artery (arrows) that extends in the retrostyloid parapharyngeal space to finally invade the skull base, as seen in (D). E, F: Patient 3, who failed treatment in level 2 lymph nodes. Contrast-enhanced CT study shows the carotid artery completely encased (arrow) in tumor. In (F), when tumor involves the carotid sheath and retrostyloid parapharyngeal space, there may be expressions of cranial neuropathies on the imaging spaces—in this case, atrophy of the right side of the tongue due to interruption of the function of cranial nerve XII. G–I: Patient 4 with a contrast-enhanced CT study showing extensive metastatic adenopathy at level 2 with a very aggressive growth pattern. In (G), the tumor invades the paravertebral space following the vertebral artery almost to the epidural space (arrows). In (H), a close-up view shows the tumor encasing the vertebral artery as it exits the uppermost transverse foramen (arrows). In (I), there is erosion of bone (arrow) by tumor tracking along the vertebral artery. J–L: Patient 5 with pharyngeal wall carcinoma and very aggressive metastatic lymphadenopathy. Contrast-enhanced CT was done. In (J), the metastatic adenopathy in the right neck diffusely infiltrates the anterior and posterior triangle and encases the entire carotid sheath as well as invades the thyroid cartilages from the external surface (arrows). There is also evidence of abnormal enhancement and probably fixation along the prevertebral fascia (arrowheads). In (K) and (L), the tumor encasing the carotid artery can be seen to follow the artery into the upper mediastinum and eventually the aortic arch origin of the brachiocephalic trunk (arrows in K and L). M: Patient 6, who failed therapy both at the primary site and in the neck for squamous cell carcinoma. The encased carotid artery lies in a zone of necrotic tumor and probably an area of chronic low-level hematoma. The wall of the carotid is very thin (arrow), and this study strongly suggests the likelihood of impending rupture of the carotid.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree