(1)
Department of Radiology, Winthrop-University Hospital, Mineola, NY, USA
Keywords
Anatomy, cervical spineBiopsy, diskBiopsy, paraspinal soft tissuesBiopsy, vertebraeCervical spine biopsy, coaxial techniqueImaging guidance, computed tomographyImaging guidance, fluoroscopyPercutaneous spine biopsy, complicationsPercutaneous spine biopsy, indicationsPercutaneous spine biopsy, contraindicationsSpine biopsy, cervicalLearning Objectives
- 1.
To understand the value and importance of radiologic anatomy as it pertains to cervical spine biopsy
- 2.
To review the indications and contraindications for cervical spine biopsy
- 3.
To learn image-guided coaxial cervical spine biopsy approaches and techniques
4.1 Introduction
Of all the percutaneous image-guided biopsy procedures that can be performed along the spinal axis, cervical spine biopsy remains the most challenging of these procedures. Indeed, many operators are reluctant to perform cervical spine procedures due to the perceived risk of the procedure in an area where the anatomy may present barriers to safe lesion access. The relatively small size of the cervical vertebrae pedicles limits the traditional “shielded” transpedicular pathway to the vertebral body for tissue sampling. The critical vascular structures of the neck, the carotid and vertebral arteries and the internal and external jugular veins, surround the anterior and lateral aspects of the cervical spine. These vascular structures, at initial inspection, may appear to prevent direct access to a vertebrae or intervertebral disk. Close proximity to other critical structures such as the lung apices, trachea, esophagus, thyroid gland and submandibular glands raises appropriate concerns for injury to these structures. The aerodigestive tract and adjacent glandular structures may also limit the access to the target lesion(s) within the cervical spine. The prominent cervical spinal cord and exiting nerve roots may also discourage attempts at sampling nearby lesions. Some operators are more comfortable with single access bone biopsy needles which make repeat passes in the neck somewhat precarious. Other operators have tried to improve their targeting using tandem needle techniques in which the biopsy needle is passed alongside a previously placed smaller gauge guide needle, but this requires a minimum of two needle passes in an area that contains numerous critical structures and, therefore, is a rarely utilized technique in the cervical spine. Cervical spine biopsy is an infrequently performed procedure when compared to thoracic or lumbar spine biopsy, hence many operators lack experience with the tools and techniques that are required to make cervical spine biopsy an effective, efficient and low risk procedure. In a single institution experience 22 cervical spine biopsies out of 703 total spine biopsies (3.1%) were performed over a period of 18 years whereas at another institution 9 out of 410 (2.2%) spine biopsies performed to assess for neoplasm, over an 8-year period, were performed in the cervical spine (Rimondi et al. 2011; Lis et al. 2004). When a procedure is performed infrequently, the comfort level with performing it decreases. The objective of this chapter is to introduce the reader to the approaches and techniques that will assist them in the performance of percutaneous image-guided cervical spine biopsy.
4.2 Anatomic Considerations
While the cervical spine consists of seven cervical vertebrae and the intervening intervertebral disks, the overall smaller size of these structures compared to the remainder of the spinal axis is associated with a relatively lower incidence of suspicious lesions in this location. The neck is bordered superiorly by the skull base and foramen magnum. Inferiorly, the neck is separated from the thoracic cavity by the thoracic inlet, including the lung apices, brachial plexus and brachiocephalic vasculature. From a biopsy perspective, with respect to approach, the neck can be divided into anterior and posterior compartments. Anterior compartment lesions will involve either the cervical vertebral bodies, intervertebral disks or the adjacent paraspinal soft tissues. Posterior compartment lesions will involve the articular masses or facet joints, the posterior elements (pedicle, laminae or spinous processes) or the adjacent paraspinal soft tissues within the perivertebral space – paraspinal component. The anterior compartment can be further subdivided into suprahyoid and infrahyoid compartments. The pertinent suprahyoid compartments include the masticator space, parapharyngeal space, perivertebral space – prevertebral component, submandibular space, retropharyngeal space and carotid space. The oropharynx and hypopharynx are located within the suprahyoid neck and are quite prominent; a breach of these structures could contaminate the spine and biopsy specimen with adverse consequences in either situation. The carotid space contains the carotid artery and the internal jugular vein, and these critical vascular structures determine the choice of approaches when considering an anterior compartment biopsy procedure (Fig. 4.1). The vertebral arteries, likewise critical vascular structures, influence the types of approaches that can be used to access the posterior compartment (Fig. 4.1). The proximity of visceral and carotid space in the infrahyoid neck will constrain the types of approaches that can be used to access the anterior cervical spine.


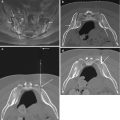
Fig. 4.1
Axial contrast-enhanced CT image (a) at C1 level shows course of vertebral arteries as they emerge from the C1 transverse foramen (large arrow) to course posterior to the lateral mass (small arrow), along a groove on the superior surface of the C1 neural arch, and then enter the spinal canal. Axial contrast-enhanced CT image (b) at C2 level shows enhancing vascular structures adjacent to lateral aspect of C2 (internal carotid artery (c; large arrow), internal jugular vein (J) and vertebral artery (V; small arrow)). If this were a biopsy case, this vascular anatomy effectively forms a barrier to biopsy needle access from these directions. The oropharynx (O) forms the anterior relation of the C2 vertebral body. Axial contrast-enhanced image (c) at C5 level shows external jugular vein branches (small arrows), internal jugular vein (J), carotid artery bifurcation on the right (large arrow) and common carotid artery (c). The vertebral artery (V) is located within the foramen transversarium. The submandibular gland (SMG) is seen anteriorly while the hypopharynx (H) forms the anterior relation at this level. Axial contrast-enhanced image (d) at the C5–6 disk space level shows external jugular vein branches (small arrows), internal jugular veins (J), common carotid arteries (C) and vertebral arteries (v). The hypopharynx (H) forms the anterior relation at this level
The carotid space is the key anatomic landmark for determining the majority of anterior-lateral or posterior-lateral approaches for image-guided percutaneous cervical spine biopsy.
Essentially, all needle passes within the anterior compartment will be made either anterior or posterior to the carotid space (Fig. 4.2). For fluoroscopy-guided procedures, the carotid space is a palpable structure – in other words, the operator can palpate the carotid pulse and use a manual displacement technique to move the carotid space out of the way prior to needle insertion. For computed tomography (CT) – guided procedures, the carotid space is readily identified, even on non-contrast CT studies of the neck. The primary objective is to determine a trajectory towards a lesion that avoids puncturing the carotid artery or jugular vein.


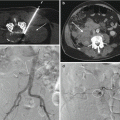
Fig. 4.2
Photographs of neck model showing a spinal needle inserted into a cervical vertebral body (a) anterior (arrow) and (b) posterior (arrow) to the carotid space
The posterior compartment of the neck includes the posterior cervical space. This includes the posterior neck muscles and the posterior elements of the cervical vertebrae. The critical anatomic structures to be aware of when attempting to biopsy lesions in this compartment are the vertebral arteries and the spinal cord. The vertebral arteries are particularly exposed in the upper cervical spine near the craniocervical junction; hence a detailed knowledge of the size and course of these vessels is mandatory when considering upper cervical spine biopsy (Fig. 4.1). The presence of a dominant vertebral artery or a hypoplastic or absent vertebral artery should be noted on the initial diagnostic studies as this will help to prevent a potential major complication. Given the small size and thickness of the posterior elements it is important to be aware of the location of the spinal cord and exiting nerve roots relative to the lesion.
A constant awareness and vigilance of the important neck compartments and critical anatomic structures is required before, during and even after the cervical spine biopsy procedure.
This is an important principle that must be adhered to when performing image-guided percutaneous spine biopsies (Ortiz et al. 2010). A detailed review of pertinent imaging studies, such as magnetic resonance imaging (MRI) studies of the cervical spine or positron emission tomography (PET) – CT examinations, is therefore mandatory prior to considering and planning a biopsy procedure. The prior study or studies should be readily available for immediate consultation during the biopsy procedure.
4.3 Indications
Image-guided percutaneous cervical spine biopsy is performed to obtain tissue samples from the cervical vertebrae (including the vertebral bodies, articular masses and posterior elements) intervertebral disks and surrounding paraspinal soft tissues. The two most common indications for performing percutaneous image-guided cervical spine biopsy include the evaluation of a neoplastic process or the assessment for possible spine infection (Table 4.1). Secondary or primary neoplastic lesions of the spine may be considered for biopsy when their histopathologic identification impacts the subsequent management of the patient. Secondary neoplastic lesions of the cervical spine include metastases, myeloma, lymphoma and leukemia; these may occur in patients with a known primary malignancy, or be the first presentation of an unknown primary tumor. Alternatively, a patient may be afflicted with two or more neoplastic conditions, and further characterization of a cervical spine mass will determine which of these processes is responsible. Primary spine tumors, though rare, do occur and may require a biopsy in order to optimize the subsequent management. Now that tailored immune-modulating therapies are available and that an analysis of cellular genomics is readily feasible, biopsies for tissue acquisition and detailed characterization are critical for both treatment planning and prognosis. A pathologic cervical vertebral compression fracture may also require a biopsy.
Table 4.1
Indications for image-guided percutaneous cervical spine biopsy
1. Secondary neoplastic involvement |
Metastasis |
Known primary neoplasm |
Two or more known primary neoplasms |
Unknown primary neoplasm |
Extension from systemic neoplasm (myeloma, lymphoma, leukemia) |
2. Primary neoplastic involvement |
3. Spine infection |
4. Spine infection mimics |
Inflammatory spondyloarthropathy (chronic hemodialysis, gout) |
5. Other |
Pathologic vertebral compression fracture |
Langerhans Cell Histiocytosis |
Evaluate recurrence of neoplasm after surgical, medical and/or radiation treatment |
Distinguishing radiation change from neoplasm |
It must be emphasized that when a neoplastic process is identified within the cervical spine, the remainder of the spinal axis and body should be evaluated for the possible presence of a more readily accessible lesion for biopsy sampling (Fig. 4.3)


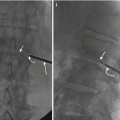
Fig. 4.3
30 year-old male with multiple spine lesions. Fat-suppressedT2 sagittal MR image of cervical spine (a) shows a C4 and T1 vertebral body lesions. The operator attempted to biopsy T1 via a posterior approach (arrow) without success (b). STIR sagittal MRI image of lumbar spine (c) shows L2 and L5 and S1 lesions. CT-guided biopsy (d) of the L2 lesion via a posterior approach (arrow) confirmed the presence of lymphoma; note the extensive retroperitoneal adenopathy (curved arrow)
In patients in whom there is an infectious or inflammatory process that involves the cervical disk space and adjacent vertebral endplate(s) a biopsy may be necessary in order to confirm the diagnosis and guide subsequent therapeutic interventions.
4.4 Contraindications
The major contraindication to performing percutaneous image-guided cervical spine biopsy is uncorrected coagulopathy (Table 4.2). Given the increased modern day use of anticoagulants and anti-platelet agents, it is imperative that the operator be aware of whether or not a patient is being treated with one or more of these medications (Refer to Chap. 2 ). A discussion with the referring clinician and patient is often required in order to determine the necessary steps in either holding or reversing the effects of these medications in order to perform the biopsy procedure. The focus of the conversation should include a risk-benefit analysis in order to ascertain the absolute need for the cervical spine biopsy procedure. The rationale for the both the cervical spine biopsy procedure and the temporary management of the coagulation status should be documented by the operator. In specific situations the patient may need to be admitted for in-hospital management of their coagulation status prior to performance of the biopsy. It must be kept in mind that the majority of these cervical spine biopsy procedures are elective or semi-urgent procedures that by default provide sufficient opportunity for adequate evaluation and communication in order to maximize the chances for a safe and effective procedure. If patient, custodial agent or administrative consent cannot be obtained then the procedure should not be performed.
Table 4.2
Contraindications to image-guided percutaneous cervical spine biopsy
Absolute |
Uncorrected coagulopathy |
Unable to obtain consent for the procedure |
Relative |
Uncooperative patient |
Unstable patient |
Suspected vascular lesion |
Small (<5 mm diameter) lesion located adjacent to critical structure |
The other contraindications to image-guided percutaneous cervical spine biopsy are relative, that is, the procedure can be performed provided that the specific management steps are taken. For example, pre-procedure catheter angiography with our without embolization may be required prior to performing a biopsy of a suspected vascular tumor such as a renal or thyroid metastasis, aneurysmal bone cyst, or aggressive hemangioma. Alternatively, a very small (<5 mm diameter) if not tiny lesion may just not be amenable to tissue sampling, especially if it is located in close proximity to the spinal cord or a vascular structure.
4.5 Risks and Complications Associated with Cervical Spine Biopsy and How to Minimize Them
The risks and complications that occur as a result of image-guided percutaneous cervical spine biopsy are uncommon and can be kept to a minimum (Wu et al. 2014). Complications that occur as a result of cervical spine biopsy include hemorrhage. As previously stated, this risk can be reduced by temporarily correcting a pre-existing coagulopathy. Identifying critical vascular structures when planning an approach to the lesion and determining an optimal trajectory will also reduce the likelihood of a hemorrhagic complication. Use of coaxial biopsy needle technology, with one needle pass and placement of a guiding cannula through the biopsy trajectory, also reduces the chances of hemorrhage. The occasional, but necessary use of intravenous contrast enhancement with CT guidance may identify subtle vascular structures that can subsequently be avoided. Pre- and peri-procedure management of suspected vascular lesions will also help to reduce the possibility of hematoma formation. Some vascular lesions may require an embolization procedure (pre-procedure endovascular embolization or post-procedure biopsy tract embolization with a small amount of surgifoam), while others can be safely sampled with fine needle aspiration techniques. Adequate blood pressure management and control during and after the biopsy procedure will also help to reduce the likelihood of hemorrhage (Fig. 4.4). There is no substitute for appropriate hand compression techniques following removal of the biopsy needle system at the puncture site, as this will help to stop bleeding at the puncture site. A few minutes (2–5 min) of manual compression will help to stabilize the biopsy tract. Bleeding into one of the neck spaces can lead to hematoma formation and possible airway compromise. Therefore, it is important to monitor the patient during and after the procedure (Fig. 4.4). Patients with hematoma formation can be imaged with CT in order to assess for stability of the hematoma and the airway.


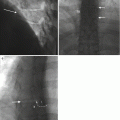
Fig. 4.4
48F for fluoroscopy guided disk aspiration at C4–5 (a). The patient’s blood pressure increased significantly during the procedure (240/120 mmHg) and she complained of difficulty swallowing. Axial CT image (a) shows acute soft swelling with hematoma formation (large arrow) and mass effect on oropharnyx (small arrow). This responded to conservative management including blood pressure control. A follow-up limited CT (b) at 1 week shows partial resolution of the swelling and residual retropharyngeal fluid (arrow)
Other procedural risks are related to needle puncture and include vascular injury, spinal cord puncture and, with lower cervical spine biopsy, lung perforation with pneumothorax formation. Fortunately with careful planning and meticulous technique, injury to these critical structures can usually be prevented. Spine infection is a potential complication of any biopsy procedure. This applies to situations where the cervical spine biopsy is being performed to evaluate for non-infectious conditions, such as neoplastic lesions of the cervical spine. The use of strict aseptic technique including shaving and hair removal at the biopsy puncture site will help to reduce the chance of infection. The use of coaxial technique, by reducing the number of times that the skin is breached, will also help to reduce the inadvertent introduction of cutaneous microbes into the deep tissues of the cervical spine. The routine use of pre-procedure intravenous antibiotic prophylaxis for the performance of cervical spine biopsy is not required unless there is a specific clinical concern that might warrant their use such as a patient that is immunocompromised. Seeding of tumor along a biopsy tract is an extremely rare complication of biopsy procedures and theoretically is even less likely to occur with coaxial biopsy techniques (Saghieh et al. 2010).
The types of complications that can occur or have been reported with cervical spine biopsy are listed in Table 4.3. It must be kept in mind that these are potential complications, many of which can be avoided if careful steps are taken to minimize the chances of their occurrence. The overall incidence of complications that can occur with skeletal biopsy is less than 0.2% (Murphy et al. 1981). The overall complication rate for percutaneous spine biopsy ranges from less than 1% to 3% (Tehranzadeh et al. 2007). A review of the literature shows that the overall incidence of complications that can occur with image-guided percutaneous cervical spine biopsy is rare (Brugieres et al. 1992; Kattapuram and Rosenthal 1987; Rimondi et al. 2008; Wu et al. 2014). The other important risk that can occur with cervical spine biopsy procedures is the possibility of a “non-diagnostic” biopsy. In this situation, tissue is obtained from the sampled lesion; nevertheless the histopathologic analysis is not able to arrive at a conclusive diagnosis. This potential outcome should always be discussed with the patient at the time of the informed consent process in order to clarify expectations and to make them aware that another biopsy procedure, percutaneous or open, might be required.
Table 4.3
Cervical spine biopsy procedure risks and complications
Hemorrhage |
Superficial – at puncture site |
Deep – potential hematoma formation – airway compromise |
Needle injury |
Artery or vein puncture |
Spinal cord puncture |
Cervical nerve root puncture |
Lung puncture – pneumothorax |
Glandular injury: thyroid gland, parathyroid gland, submandibular gland, parotid gland
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|